Clinical pathology Dr S Homathy 1 Pathology The








































































































- Slides: 132

Clinical pathology Dr S Homathy 1

Pathology • The branch of medicine dealing with the essential nature of disease, especially changes in body tissues and organs that cause or are caused by disease. • The discipline of Pathology includes both Clinical and Anatomic Pathology. 2

• Clinical pathology – pathology applied to the solution of clinical problems, especially the use of laboratory methods in clinical diagnosis. 3

• It is vital for educating physicians to order the correct tests and understand the clinical significance of test results. • The clinician depend upon the laboratory for the diagnosis of diseases • The laboratory plays a crucial role in the diagnosis of disease and therapeutic management of patients. 4

• It is the key subject in the studies of paramedical sciences • It forms bridge between – the preclinical sciences of anatomy, physilogy and biochemistry on one hand – clinical branches of medical and surgical disciplines on the other 5

• The diagnosis of a disease involves examination of specimens collected from the patients include – urine, – stool, – sputum, – blood, – CSF, – other body fluids and – biopsy material. 6

• The laboratory study of disease by a pathologist using techniques appropriate to the specimen being studied. 7

Major subspecialty areas in Clinical Pathology are: • • • • Clinical Chemistry/Toxicology Coagulation Cytogenetics Flow Cytometry Haematology Haematopathology Microbiology Molecular Diagnostics Transfusion Medicine Bacteriology, Chemistry, and Serology. Molecular genetics pathology. In some countries other subspecialties fall under certified Clinical Biologists responsibility – Assisted reproductive technology, Sperm bank and Semen analysis • Immunopathology 8

• What are the sections of the lab and their functions? 9

Tools of Clinical Pathology • Microscopes, analysers, strips, centrifugal machines. . . Macroscopic examination • The visual examination of the taken liquid is a first main indication for the pathologist or the physician. 10

Microscopical examination • Microscopic analysis is an important activity of the pathologist and the laboratory assistant. • They have many different colourings at their disposal (H&E, Gram, Grocott, Ziehl-Neelsen, …). • Immunofluorescence, cytochemistry, the immunocytochemistry and FISH are also used in order make a correct diagnosis. • This stage allows the pathologist to determine the character of the liquid: – – “normal”, tumoral, Inflammatory even infectious. 11

Analyzers • The analysers, by the association of robotics and spectrophotometry, – allowed these last decades a better reproducibility of the results of proportionings, – in particular in medical biochemistry and hematology. • in -vitro diagnosis include chains of automats, – i. e. a system allowing the automatic transfer of the tubes towards the various types of automats of the same mark. • These systems can include the computer-assisted management of a serum library. • These analysers must undergo daily controls to guarantee a result just possible, one speaks about quality control. • These analysers must also undergo daily, weekly and monthly maintenances. 12

Cultures • A big part of the examinations of clinical pathology, primarily in medical microbiology, use culture media. • Those allow, for example, the description of one or several infectious agents responsible of the clinical signs. 13

• Values known as “normal” or reference values 14

Quality • Laboratories can only produce quality results on quality samples • And on quality requests • Rubbish In - Rubbish Out • Ideal sample mimics the in vivo state 15

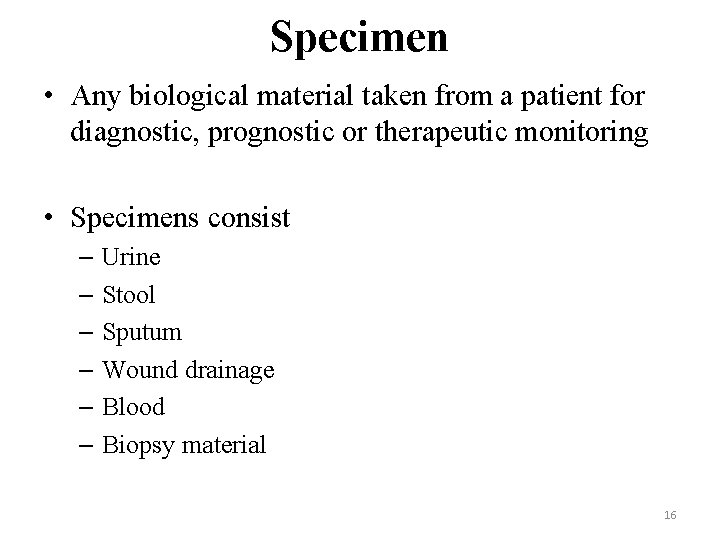
Specimen • Any biological material taken from a patient for diagnostic, prognostic or therapeutic monitoring • Specimens consist – Urine – Stool – Sputum – Wound drainage – Blood – Biopsy material 16

• All samples must be considered to be infectious – Use of “ Universal precautions” handling – Never assume any sample is “ safe” 17

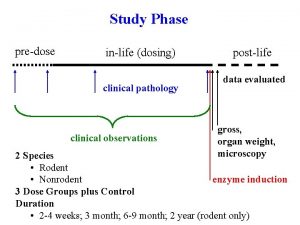
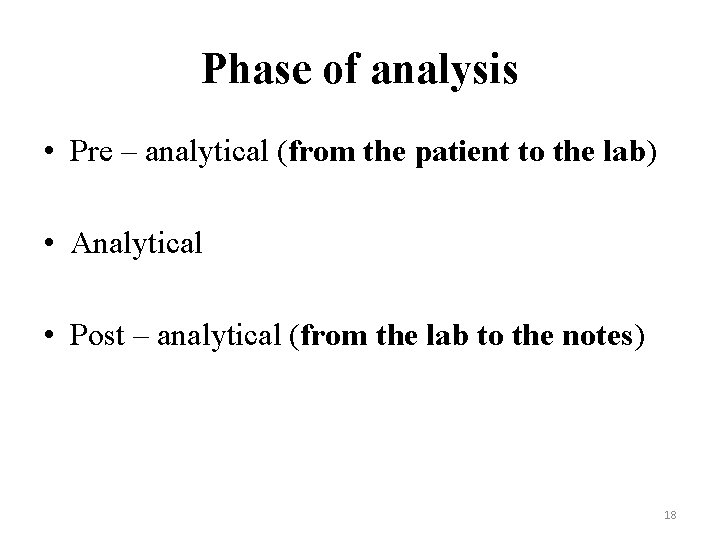
Phase of analysis • Pre – analytical (from the patient to the lab) • Analytical • Post – analytical (from the lab to the notes) 18

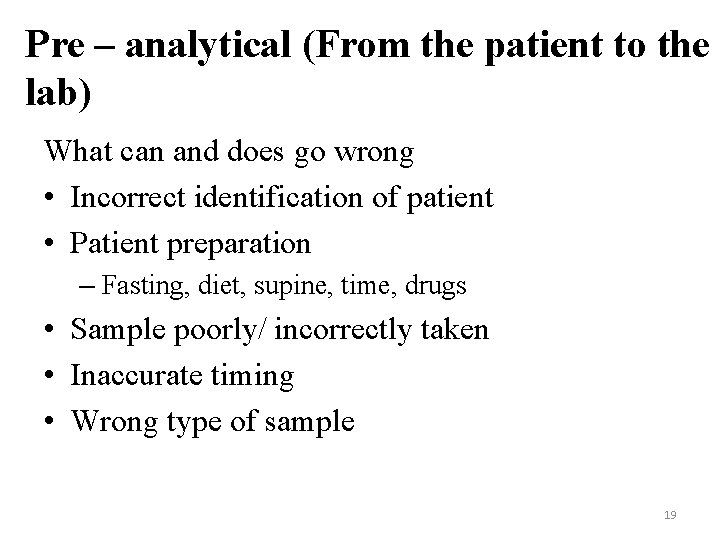
Pre – analytical (From the patient to the lab) What can and does go wrong • Incorrect identification of patient • Patient preparation – Fasting, diet, supine, time, drugs • Sample poorly/ incorrectly taken • Inaccurate timing • Wrong type of sample 19

• • • Incorrect containers Under- filling Mislabelling/ no labelling Incorrect storage / transport (ice, warm, delay) Loss, breakage 20

The patient • Do patients always disclose history? – Confused – Frightened – In pain – Want to help – Any previous diagnosis available 21

Patient preparation • Prior to each collection, – review the appropriate test description, – including the specimen type to be collected, – the volume, – the procedure, – the collection materials, and – the storage and handling instructions 22

• Proper sample collection and handling is an integral part of obtaining a valid and timely laboratory test result 23

• Specimens must be obtained using – proper phlebotomy techniques, – collected in the proper container, – correctly labeled (in the presence of the patient) and – promptly transported to the laboratory. • It is the policy of the laboratory to reject samples when there is failure to follow these guidelines. • All specimens should be handled with universal precautions, as if they are hazardous and infectious. 24

Blood collection • As patients to identify themselves • When blood taken write all relevant details on collection tubes immediately 25

Other information should include • Ward • Date of collection • Time of collection e. g: cortisol 26

Patient identification • All patients from whom clinical specimens are obtained must be positively identified prior to specimen collection. • Positive identification is the responsibility of the person collecting the sample. 27

• Identify the patient prior to sample collection, using at least two patient identifiers. – Verify the patient’s name, – unit history number on the identification armband (inpatients), or – drivers’ license or other ID (outpatients) with the information on the requisition. 28

SPECIMEN COLLECTION TIMING • The basal state (the early morning approximately 12 hours after the last ingestion of food) is recommended for determining the concentration of body constituents such as – glucose, – cholesterol, – triglycerides, – electrolytes, and – proteins. 29

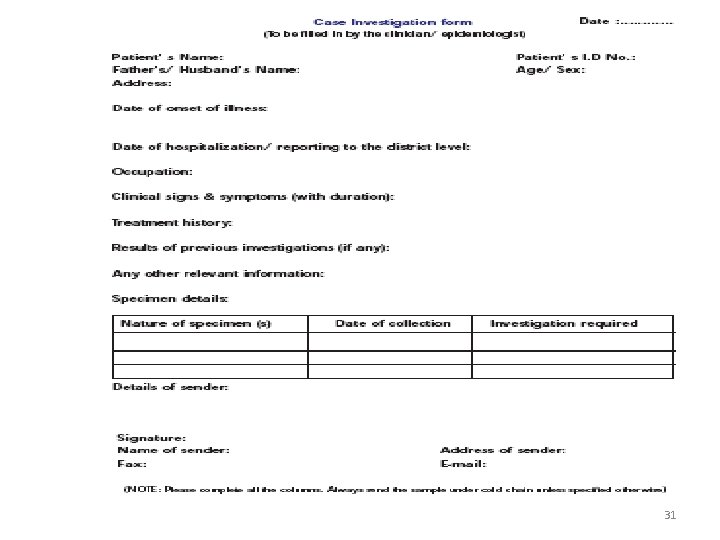
Test request • The test requisition must include: – the patient's name – a unique identification number which assures positive patient identification (e. g. , BHT number) – the unit / clinic location – The name(s) and signature of the authorized provider requesting the test(s) – the test(s)/procedure(s) being ordered – the date and time of specimen collection is required on all requisitions – name or initials of person collecting specimen – pager/ phone number of appropriate contact person – office address of requesting physician – diagnosis: ICD 9 code or narrative description 30

31

Specimen collection • Prioritize collection – "STAT" means special turnaround time and must be collected immediately. – It may involve a patient whose medical condition has suddenly become very critical and must be treated as a medical emergency. • Fasting: Requests for "fasting" specimens are performed before routine requests, so patients can eat meals on schedule. 32

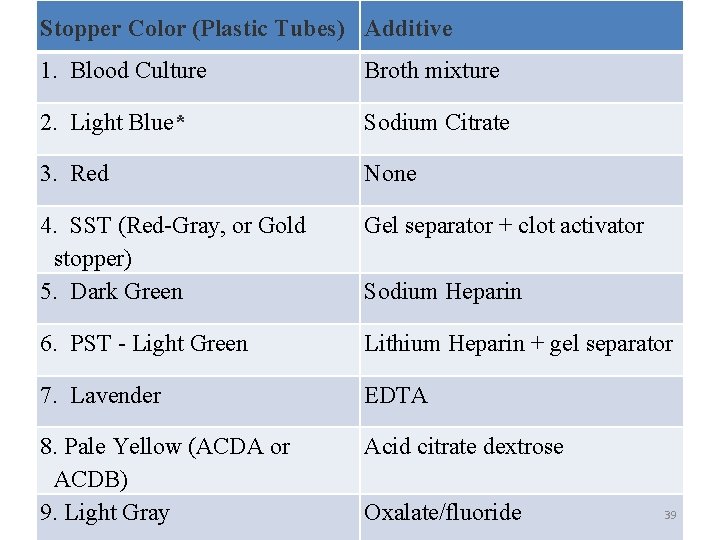
Order of draw • Blood collection tubes must be drawn in a specific order to avoid cross-contamination of additives between tubes. The recommended order of draw for plastic vacutainer tubes is: • First - – blood culture bottle or tube (yellow or yellowblack top) 33

• Second - – coagulation tube (light blue top). – If just a routine coagulation assay is the only test ordered, then a single light blue top tube may be drawn. – If there is a concern regarding contamination by tissue fluids or thromboplastins, then one may draw a non-additive tube (red top) first, and then the light blue top tube. 34

• Third - – non-additive tube (red top) • Last - additive tubes in this order: – SST (red-gray or gold top): Contains a gel separator and a clot activator. – Sodium heparin (dark green top) – PST (light green top): Contains lithium heparin anticoagulant and a gel separator. – EDTA (lavender top) – ACDA or ACDB (pale yellow top): Contains acid citrate dextrose. – Oxalate/fluoride (light gray top) 35

• Tubes with additives must be filled to their stated volumes and thoroughly mixed. • Erroneous test results may be obtained when the tube is not filled to the stated volume and blood is not thoroughly mixed with the additive. • Mix all tubes with anticoagulant by gentle inversion for 15 seconds. 36

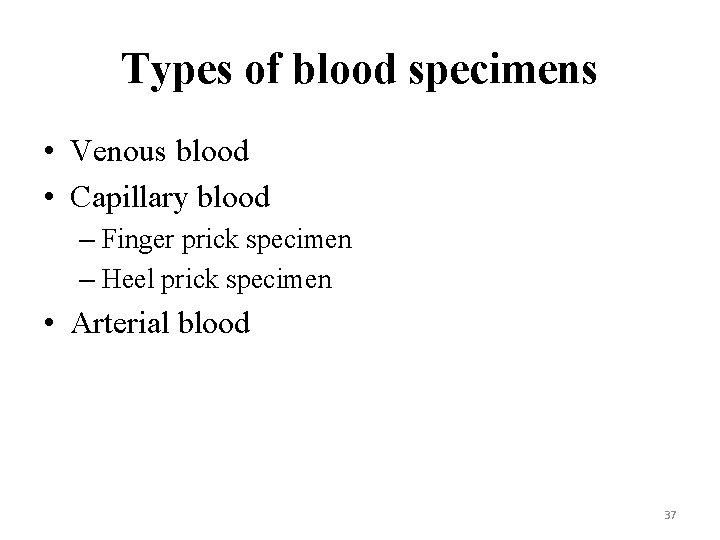
Types of blood specimens • Venous blood • Capillary blood – Finger prick specimen – Heel prick specimen • Arterial blood 37

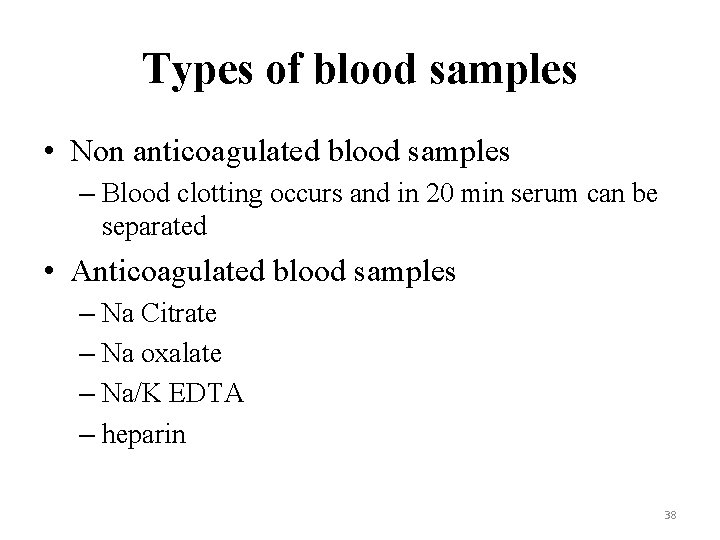
Types of blood samples • Non anticoagulated blood samples – Blood clotting occurs and in 20 min serum can be separated • Anticoagulated blood samples – Na Citrate – Na oxalate – Na/K EDTA – heparin 38

Stopper Color (Plastic Tubes) Additive 1. Blood Culture Broth mixture 2. Light Blue* Sodium Citrate 3. Red None 4. SST (Red-Gray, or Gold stopper) 5. Dark Green Gel separator + clot activator 6. PST - Light Green Lithium Heparin + gel separator 7. Lavender EDTA 8. Pale Yellow (ACDA or ACDB) 9. Light Gray Acid citrate dextrose Sodium Heparin Oxalate/fluoride 39

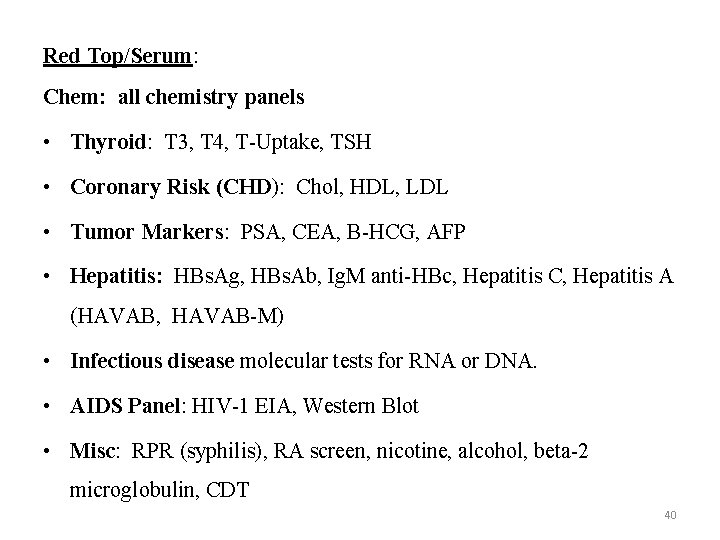
Red Top/Serum: Chem: all chemistry panels • Thyroid: T 3, T 4, T-Uptake, TSH • Coronary Risk (CHD): Chol, HDL, LDL • Tumor Markers: PSA, CEA, B-HCG, AFP • Hepatitis: HBs. Ag, HBs. Ab, Ig. M anti-HBc, Hepatitis C, Hepatitis A (HAVAB, HAVAB-M) • Infectious disease molecular tests for RNA or DNA. • AIDS Panel: HIV-1 EIA, Western Blot • Misc: RPR (syphilis), RA screen, nicotine, alcohol, beta-2 microglobulin, CDT 40

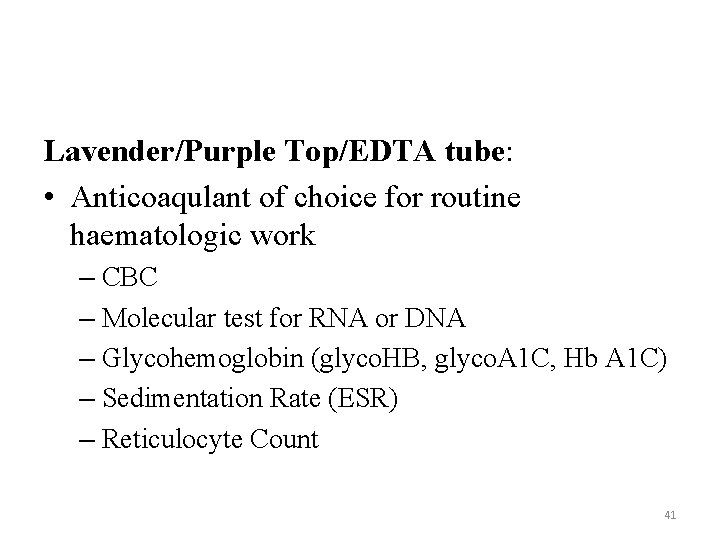
Lavender/Purple Top/EDTA tube: • Anticoaqulant of choice for routine haematologic work – CBC – Molecular test for RNA or DNA – Glycohemoglobin (glyco. HB, glyco. A 1 C, Hb A 1 C) – Sedimentation Rate (ESR) – Reticulocyte Count 41

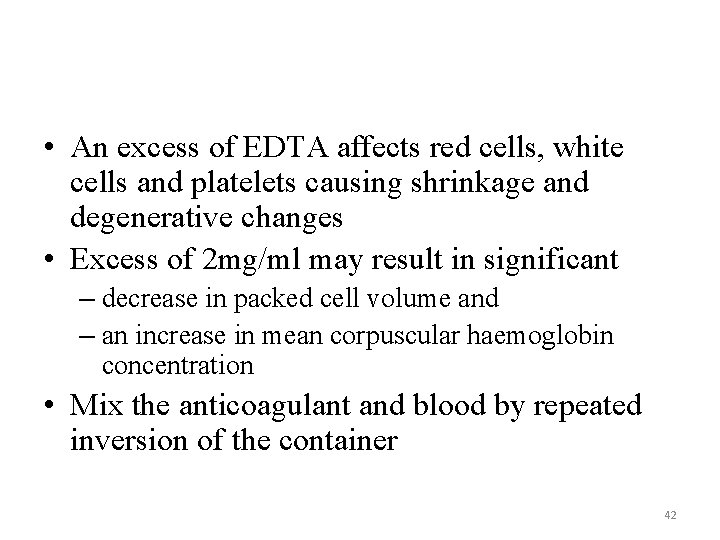
• An excess of EDTA affects red cells, white cells and platelets causing shrinkage and degenerative changes • Excess of 2 mg/ml may result in significant – decrease in packed cell volume and – an increase in mean corpuscular haemoglobin concentration • Mix the anticoagulant and blood by repeated inversion of the container 42

Blue Top/Sodium Citrate: • PT • APTT • Fibrinogen 43

Gray Top/Sodium Fluoride: • Glucose, Blood Alcohol Urine: • Urinalysis • Drug Screen 44

Heparin • Used in the osmotic fragility test and for immuno phenotyping • Should not be used to prepare blood films due to faint blue coloration to the background. • Do not use this for WBC as it causes WBC to clump • It does not affect RBC size and less likely to cause haemolysis than EDTA 45

Tri sodium citrate • It is the choice for coagulation studies • This is the anticoagulant for ESR – 1 volume of sodium citrate is mixed with 4 volumes of blood 46

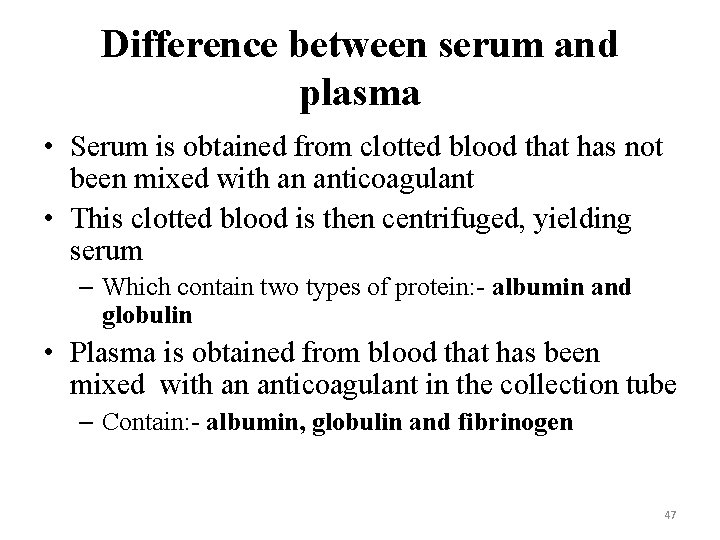
Difference between serum and plasma • Serum is obtained from clotted blood that has not been mixed with an anticoagulant • This clotted blood is then centrifuged, yielding serum – Which contain two types of protein: - albumin and globulin • Plasma is obtained from blood that has been mixed with an anticoagulant in the collection tube – Contain: - albumin, globulin and fibrinogen 47

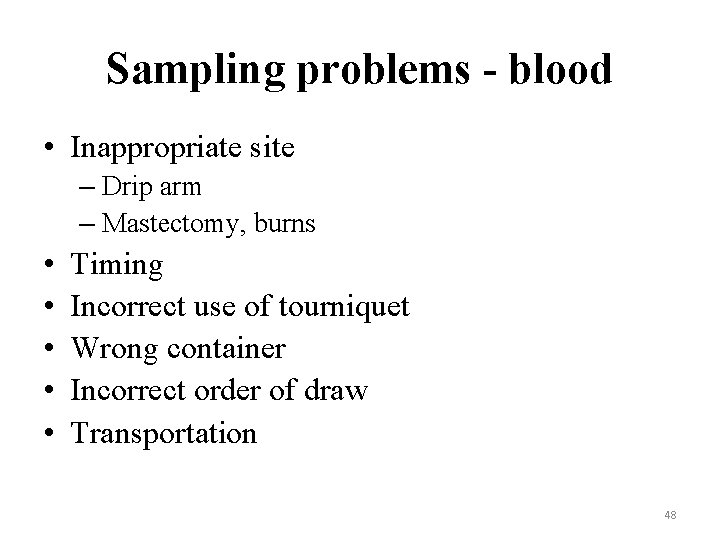
Sampling problems - blood • Inappropriate site – Drip arm – Mastectomy, burns • • • Timing Incorrect use of tourniquet Wrong container Incorrect order of draw Transportation 48

Urine collection • 24 hour sample must include all urine passed in this period • If less than 24 h, inform the lab • Mid stream sample – self explanatory • Early morning sample- often best • Correct container type 49

Urine samples • • Incorrect timing Inappropriate for test required Sterility volume 50

Other consideration • Swabs for culture may need specific transport media e. g: clamydia • Blood cultures- special bottles and technique for taking the blood • Tissue for histology- fixative – Extreme care needed when using formalin 51

The contribution of the ward staff to valid laboratory results • Providing correct and adequate information to the laboratory staff • Correct patient preparation for test and providing correct advice to patients on preparation • Collection of specimens by the correct technique at the appropriate time • Correct labeling • Speedy delivery to the lab 52

General specimen collection • Some of the common considerations affecting all types of specimens include – Insufficient quantity of specimen to run test – Failure to use correct container – Inaccurate patient instruction prior to collection – Leakage and contamination of specimens 53

Other factors affecting laboratory results Exercise • Moderate exercise can cause an increase in – Blood glucose – Lactic acid – Serum protein – CK 54

Emotional / physical stress Time of day • Diurnal variations and variations in circadian rhythm can also affect test results – GH peaks in the morning before waking and decreases throughout the day – Serum iron levels may change as much as 30% to 50% , depending on individual variation, from morning until evening 55

Timed specimens • Two types of timed specimen – One is for a single blood specimen ordered to be drawn at a specific time – Other is for a test that may require multiple blood specimens to be collected at several specific times 56

• Types of anticoagulants – EDTA – Citrate – Heparin – Oxalate 57

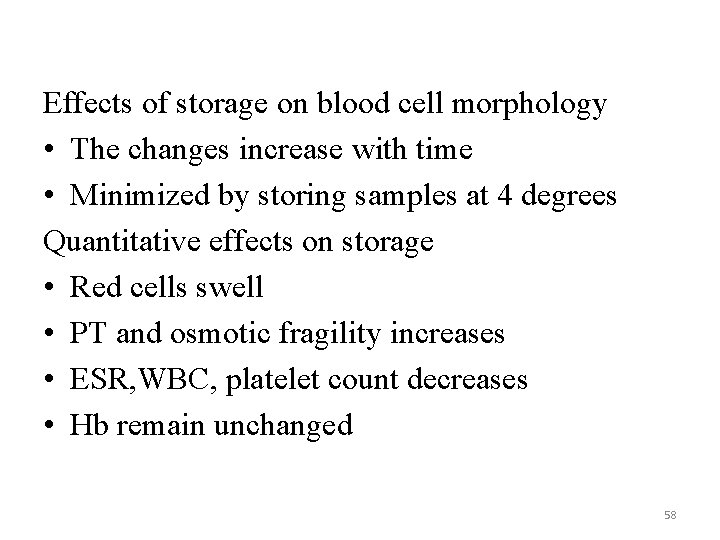
Effects of storage on blood cell morphology • The changes increase with time • Minimized by storing samples at 4 degrees Quantitative effects on storage • Red cells swell • PT and osmotic fragility increases • ESR, WBC, platelet count decreases • Hb remain unchanged 58

Specimen transport and storage • All laboratory specimens shall be placed in leakproof containers (i. e. , culturettes, vacuum tubes), • then bagged in single, biohazard specimen bags. • Place the requisition slip in the outside pocket of the biohazard specimen bag. • Tubed Specimens: Specimens may be sent through the tube system 59

• To ensure the validity of test results and the safety of laboratory personnel, – specimens that leak in transit will be discarded, – and the sender notified to resend another sample 60

Spill Cleanup • If a pneumatic capsule is received that appears grossly wet or soiled, • wear gloves before handling the capsule and removing the contents. • Be aware that there may be broken glass or plastic inside! • Remove sharp objects (broken glass or plastic) using forceps. • Discard any wet or soiled padding as infectious waste. • Clean the inside and outside surfaces of the pneumatic capsule with a hospital-grade disinfectant. • Call the physical plant dispatcher and notify them of the contamination of the tube system 61

The Basics of Specimen Collection and Handling of Urine Testing Types of Collection • Laboratory urine specimens are classified – by the type of collection conducted or – by the collection procedure used to obtain the specimen. 62

Random Specimen • This is the specimen most commonly sent to the laboratory for analysis, • Primarily, it is the easiest to obtain and is readily available. • This specimen is usually submitted for urinalysis and microscopic analysis, • although it is not the specimen of choice for either of these tests. • Random specimens can sometimes give an inaccurate view of a patient's health if the specimen is – too diluted and – analyte values are artificially lowered. 63

• Pediatric specimens, which routinely undergo chemistry and microscopic analysis, are generally of this type. • As the name implies, the random specimen can be collected at any time. • Although there are no specific guidelines for – how the collection should be conducted, – avoiding the introduction of contaminants into the specimen is recommended. • This requires explicit instructions to patients so that they do not touch the inside of the cup or cup lid 64

First Morning Specimen • This is the specimen of choice for urinalysis and microscopic analysis, – since the urine is generally more concentrated (due to the length of time the urine is allowed to remain in the bladder) and, – therefore, contains relatively higher levels of cellular elements and analytes such as protein, if present. – Also called an 8 -hour specimen, the first morning specimen is collected when the patient first wakes up in the morning, having emptied the bladder before 65 going to sleep.

– Since the urine can be collected over any eighthour period, collection is practical for patients who have atypical work/sleep schedules. – Proper collection practices and accurate recording of the collection time are important criteria of a first morning specimen. – Any urine that is voided from the bladder during the eight-hour collection period should be pooled and refrigerated, so that a true 8 -hour specimen is obtained 66

Midstream Clean Catch Specimen • This is the preferred type of specimen for culture and sensitivity testing – because of the reduced incidence of cellular and microbial contamination. • Patients are required to first cleanse the urethral area. • The patient should then void the first portion of the urine stream into the toilet. • These first steps significantly reduce the opportunities for contaminants to enter into the urine stream. • The urine midstream is then collected into a clean container (any excess urine should be voided into the toilet). • This method of collection can be conducted at any time of day or night. 67

Timed Collection Specimen • Among the most commonly performed tests requiring timed specimens are those measuring – – – creatinine, urine urea nitrogen, glucose, sodium, potassium, or analytes such as catecholamines and 17 -hydroxysteroids that are affected by diurnal variations. • A timed specimen is collected to measure the concentration of these substances in urine over a specified length of time, – usually 8 or 24 hours. 68

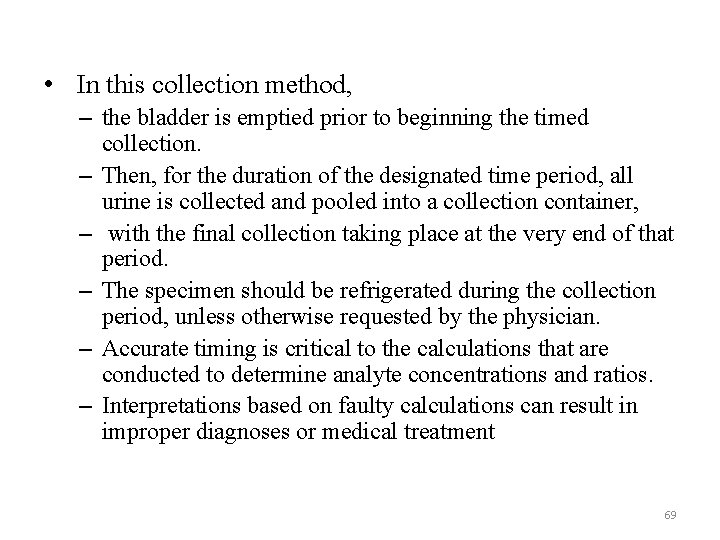
• In this collection method, – the bladder is emptied prior to beginning the timed collection. – Then, for the duration of the designated time period, all urine is collected and pooled into a collection container, – with the final collection taking place at the very end of that period. – The specimen should be refrigerated during the collection period, unless otherwise requested by the physician. – Accurate timing is critical to the calculations that are conducted to determine analyte concentrations and ratios. – Interpretations based on faulty calculations can result in improper diagnoses or medical treatment 69

Catheter Collection Specimen • This assisted procedure is conducted when a patient is bedridden or cannot urinate independently. • The healthcare provider inserts a foley catheter into the bladder through the urethra to collect the urine specimen. • Specimens may also be collected through an existing foley catheter. • Specimens may be collected directly from a foley into an evacuated tube or transferred from a syringe into a tube or cup. . 70

Suprapubic Aspiration Specimen • This method is used when a bedridden patient cannot be catheterized or a sterile specimen is required. • The urine specimen is collected by needle aspiration through the abdominal wall into the bladder. Pediatric Specimen • For infants and small children, a special urine collection bag is adhered to the skin surrounding the urethral area. • Once the collection is completed, the urine is poured into a collection cup or transferred directly into an evacuated tube with a transfer straw. • Urine collected from a diaper is not recommended for laboratory testing – since contamination from the diaper material may affect test results 71

Urine Collection Containers (cups for collection and transport) • Urine collection container cups come in a variety of shapes and sizes with lids that are either snap on or screw on. • To protect healthcare personnel from exposure to the specimen and protect the specimen from exposure to contaminants, – leak-resistant cups should be utilized. • Some urine transport cup closures have special access ports that allow closed-system transfer of urine directly from the collection device to the tube. 72

Urine Collection Containers (24 -hour collection) • Urine collection containers for 24 -hour specimens come in a variety of shapes and colors, – but most are of 3 liter (L) capacity and – are amber colored • to protect light-sensitive analytes such as porphyrins and urobilinogen. • Closure types vary and some have a port for ease of specimen transfer into a tube. • When a preservative is required, it should be added to the collection container before the urine collection begins and warning labels should be placed on the container. 73

• If there is more than one acceptable preservative for the analyte being tested, the least hazardous one should be selected. • A corresponding Material Safety Data Sheet (MSDS) should be given to the patient, and the healthcare provider should explain any potential hazards. • Some common 24 -hour preservatives are – – hydrochloric acid, boric acid, acetic acid and toluene 74

Urinalysis Tubes • Urine specimens are poured directly into urinalysis tubes with screw - or snap-on caps. • Additionally, there are evacuated tubes similar to those used in blood collection that are filled by using a straw device, from cups with integrated transfer devices built into their lid, or from direct sampling devices that are used to access catheter sampling ports. • Urinalysis tubes come in an array of tube shapes: conical bottom, round bottom, or flat bottom. • Conical bottom tubes provide the best sediment collection for microscopic analysis. • Some tubes are specially designed to be used with a pipetter that allows for standardized sampling. • The tubes you select should be able to withstand centrifugation and, • if used with an automated instrument system, – should be compatible with the corresponding racks and carriers. – Fill volumes of urinalysis tubes usually range from 8 to 15 m. L 75

Preservatives for Urinalysis • NCCLS Guidelines recommend testing urine within two hours of its collection. • However, refrigeration or chemical preservation of urine specimens may be utilized – if testing or refrigeration within a two-hour window is not possible • A variety of urine preservatives (tartaric and boric acids being the most common) are available that allow urine to be kept at room temperature while still providing results comparable to those of refrigerated urine. • Generally, the length of preservation capacity ranges from 24 to 72 hours. 76

• Claims for the length of specific analyte preservation should be obtained from the manufacturer. • When a specimen is directly transferred from a collection cup into a preservative tube, – it provides – a stable environment for the specimen until testing can be conducted and – reduces the risk of bacterial overgrowth or specimen decomposition. • Non-additive tubes (those not containing any chemical preservatives) can be used for urinalysis, – but must be handled following strict timing and refrigeration guidelines 77

Preservatives for Culture and Sensitivity (C&S) Testing • The most common preservative used for culture and sensitivity is boric acid, – which comes in tablet, powder or lyophilized form. • There is clinical evidence to suggest that non-buffered boric acid may be harmful to certain organisms and – that buffered boric acid preservatives can reduce the harmful effects of the preservative on the organisms. – Preserved urine specimens can be stored at room temperature until time of testing. – Product claims regarding duration of preservative potency should be obtained from the particular manufacturer 78

Measuring chemical properties of urine=Urinalysis • • Glucose Ketones Protein Blood- haematuria p. H Specific gravity Microscopic examination 79

Serum Collection Venous blood in sterile test tube • let clot for 20 - 30 minutes at ambient temperature • glass better than plastic Handling Place at 4 -8 o. C for clot retraction for at least 1 -2 hours Centrifuge at 1 500 RPM for 5 -10 min • separates serum from the clot 80

• Transport – 4 -8 o. C if transport lasts less than 10 days – Freeze at -20 o. C if storage for weeks or months before processing and shipment to reference laboratory • Avoid repeated freeze-thaw cycles • destroys Ig. M • To avoid hemolysis: do not freeze unseparated blood 81

Specimen Collection and Transport Guidelines • As with any type of laboratory specimen, there are certain criteria that need to be met for proper collection and transportation of urine specimens. • This will ensure proper stability of the specimen and more accurate test results. • All urine collection and/or transport containers should be clean and free of particles or interfering substances. • The collection and/or transport container should have a secure lid and be leak-resistent. • Leak-resistent containers reduce specimen loss and healthcare worker exposure to the specimen while also protecting the specimen from contaminants. 82

• It is good practice to use containers that are made of break-resistant plastic, which is safer than glass. • The container material should not leach interfering substances into the specimen. • Specimen containers should not be reused. • The NCCLS guidelines for urine, GP-16 A 2, recommend the use of a primary collection container that holds at least 50 m. L, has a wide base and an opening of at least 4 cm. • The wide base prevents spillage and a 4 cm opening is an adequate target for urine collection. • The 24 -hour containers should hold up to 3 L. 83

• The NCCLS guidelines recommend sterile collection containers for microbiology specimens. • • The containers should have secure closures to prevent specimen loss and to protect the specimen from contaminants. • Transport tubes should be compatible with automated systems and instruments used by the lab. • Collection containers and/or transport tubes should be compatible with the pneumatic tube system if one is used for urine specimen transport in the facility. • A leak-proof device in this situation is critical. • NCCLS recommends the use of an amber colored container for specimens being assayed for light sensitive analytes, such as urobilinogen and porphyrins. • The colorant prevents the degradation of certain analytes. • Proper labeling should be applied to the collection container or tubes 84

Phlebotomy 1) Wash hands thoroughly before beginning any phlebotomy procedure. • Be sure to check expiration dates on tubes before proceeding. • DO NOT USE EXPIRED TUBES. 2) Confirm the identity of the patient by checking at least two identifiers before collecting the specimen(s). • This can be done by asking the patient to state their full name and requesting to see the patient's driver's license to verify picture, name, date of birth and/or drivers license number and documenting the information on the 85 consent/chain of custody form.

3) Explain the procedure, including small risk of hematoma, slight pain, and some lightheadedness. • Inquire whether the patient has a history of fainting or dizziness with phlebotomy procedures so that ammonia inhalants can be obtained if necessary. • Explain that loss of vacuum or a collapsed vein may necessitate another draw. 4) On a table or desk, assemble all necessary equipment: cotton balls and/or gauze, tubes, safety needle, alcohol swab, tourniquet, gloves, and bandaid. • Wearing safety gloves is MANDATORY. • Wear additional protective equipment if contamination is expected. • Safety needles should always be used; the only exception is if the patient is very hard to draw then a butterfly needle set may be used. 86

5) Position the patient so that they are seated comfortably in a chair with their arm extended on an armrest, desk, or table to form a straight line from the shoulder to the wrist. • The patient’s arm and elbow should be firmly supported, and not bent at the elbow. 6) Check both arms to select the larger and fuller veins. • Palpate and trace the path of the veins several times with your index finger. • Tap the vein at the site of the draw with your index finger and second finger. • This will cause the vein to dilate. 87

The following factors should be considered in site selection: • i) Extensive scarring. – Healed burn areas or scar tissue should be avoided. • ii) Specimens collected from an area with a hematoma may yield erroneous test results. – If another vein site is not available, the specimen should be collected distal to the hematoma. 88

7) Apply the tourniquet. 8) Ask the patient to open and close his/her fist so their veins become prominent. • Vigorous hand pumping is not necessary to activate blood flow and should be avoided. 9) Clean the venipuncture site with the alcohol swab in a circular motion from the center of the area to the outside. – Allow the area to air-dry to prevent haemolysis and a burning sensation to the patient. 89

10) Insert the stopper of the first tube to be drawn into the adaptor. • Do not push too far to avoid premature loss of vacuum via puncture of the needle. • The recommended order of draw when drawing more than one tube is as follows: ♦ Non additive tube (red stopper) ♦ Coagulation tube (light blue stopper) ♦ Serum separator tube (SST) or serum tube ♦ Additive tube (lavender stopper, green stopper, etc) 90

11) Insert the needle into the vein with the bevel facing upward. – Puncture the stopper on the tube by pushing it onto the end of the needle, and grasp the edge of the adaptor to provide stability once the blood flow has begun. • Have the patient open his/her fist. 12) Fill the tube until the vacuum is exhausted. • Remove the tube from the adaptor and insert subsequent tubes. • Be sure that all tubes are completely filled to ensure sufficient blood sample for laboratory analysis. . 91

13) Place a cotton ball or 2 x 2 square piece of gauze over the site. – All used needles must be disposed of in a puncture proof biohazard receptacle. – Never recap a needle. – – – Recapping, purposeful bending, breaking, removing from disposable syringes, or other manual manipulations of needles • is prohibited. • Apply pressure to the site for 2 -5 minutes. • Place a bandaid over the puncture site. 14) Again verify that the information on the sample tubes match the consent/requisition form 92

15) Remove gloves and dispose of in a properly identified biohazard bag or container. – Wash hands thoroughly after phlebotomy. 93

Additional venipuncture consideration 1) Prevention of Hematoma: • a) Puncture only the uppermost wall of the vein • b) Release the tourniquet before removing the needle from the vein. • c) Use only major veins; not superficial veins • d) Make sure that the needle fully penetrates the uppermost wall of the vein. • Partial penetration may allow blood to leak into the soft tissue surrounding the vein by way of the needle bevel. • e) Apply a small amount of pressure to the area with the 94

2) Prevention of Hemolysis: • a) Mix anticoagulated specimens thoroughly by inverting each tube gently 8 to 10 times. – Do not shake. – Vigorous mixing may cause hemolysis. • b) Avoid drawing blood from an area with a hematoma. • c) Ascertain that the venipuncture site is dry without touching it. 95

3) If a Blood Sample is Unobtainable • a) Change the position of the needle. – If the needle has penetrated too far into the vein, pull it back slightly. – If it has not penetrated far enough, advance it farther into the vein. – Rotate the needle a half-turn. • b) Try another tube; the tube may not have sufficient vacuum. • c) Loosen the tourniquet. – It may be applied too tightly, thereby stopping the blood flow. – Reapply the tourniquet loosely. – This procedure can be accomplished easily when using the velcro-type tourniquet by releasing it and quickly pressing it together again. • d) Probing for the vein is NOT recommended as it is painful to the patient. In most cases, another puncture in a site below the first site is advised • e) Never attempt a venipuncture more than twice. – Have another person attempt to draw the specimen 96

Blood for smears Collection Capillary blood from finger prick • make smear • fix with methanol or other fixative Handling and transport Transport slides within 24 hours Do not refrigerate (can alter cell morphology) 97

Fingerstick Collection Instructions • 1) Have the applicant wash his/her hands in warm, soapy water. – Rinse and dry completely. • 2) Thoroughly clean site of skin puncture with alcohol swab and allow finger to air dry. – Remove collection card from envelope. • 3) Puncture finger firmly with lancet near the tip but slightly to the side. – It is important to obtain a free flow of blood without excessive squeezing, as this may dilute the blood with tissue fluid. 98

• 4) With the applicant’s/donor’s arm slanting downward, palm upright, add drops of blood to the small rectangular section of the card until the strip turns red at the first (dark) line. – Do not touch the collection strip in the middle of the card. – Lay the card on a clean, dry surface while placing blood on the card 99

• 5) When the card is filled per above, apply pressure to fingertip with alcohol swab until bleeding stops. • 6) Place adhesive bandage over puncture site. • 7) Do not attempt to re-cap lancet. – Place used lancet in lancet disposal tube, dispose of lancet – NEVER RETURN A USED LANCET! • 8) Allow blood on card to air dry for at least 20 -30 minutes. Place card into Blood Sample – Return Bag and seal. – Place the signed security seal from the consent over sealed end of Sample Return Bag. • 9) PLEASE BE SURE TO INCLUDE THE SIGNED AUTHORIZATION WHEN SENDING SPECIMENS TO THE LAB. 100

101

Bacteriology specimen collection Collection of Specimens for Culture: General Information • 1) Labeling. – Appropriate information is critical to proper processing of test requests. • Although pertinent clinical information is highly desirable, if it is not available, please provide at least the following information – a) Patient’s name and second unique patient identifier – b) Source of specimen or collection site – c) Date – d) Specimen – e) Test desired 102

• 2) Obtain specimen correctly. – a) Explain completely to the patient. – b) Use a sterile container – c) Label correctly and send the specimen to the laboratory promptly – d) Avoid contamination of the container 103

• 3) Timing of collection – a) Sputum, urine, stool, etc. are best collected in early morning and sent to the laboratory the same day. 104

b) Blood Collection Venous blood • infants: 0. 5 – 2 ml • children: 2 – 5 ml • adults: 5 – 10 ml Requires aseptic technique Collect within 10 minutes of fever • if suspect bacterial endocarditis: 3 sets of blood culture 105

• i) A blood culture requires two bottles of blood — one for aerobic and one for anaerobic culture. • ii) Collect blood specimens before treatment is initiated, if possible. • iii) Collect two or three sets early in the illness; repeat if they are negative after 48 hours of growth – Each blood culture should be collected from a separate venipuncture 106

• iv) Organisms are continuously shed during intravascular infections, such as – endocarditis, but they are intermittently shed during occult infections. – In some instances of occult infection, there is a predictable fever pattern. – If this is the case, the blood for culture is best collected 30 minutes prior to the fever spike. • v) The yield beyond three or four cultures is minimal in most circumstances, and collection of more than this is discouraged. 107

Handling and Transport Collect into bottles with infusion broth • change needle to inoculate the broth Transport upright with cushion • prevents hemolysis Wrap tubes with absorbent cotton Travel at ambient temperature Store at 4 o. C if can’t reach laboratory in 24 h 108

Obtaining culture specimens from the nasopharyngeal area and the throat. • 1) A nasopharyngeal culture is obtained by inserting a thin sterile swab gently through the nose to touch the pharynx; gently rotate and remove. • 2) A throat culture is obtained by introducing a sterile swab into the mouth. • Use a tongue blade to avoid contaminating the specimen with oral secretions. • Firmly swab both tonsilar fossae, posterior pharynx, and any inflamed or ulcerated areas. 109

Lower Respiratory Tract Sputum. • sputum cultures, • including such alternatives as – induced sputum, tracheal aspiration, and bronchial washings. 1) Rinsing the mouth with saline or water (but not mouthwash) may reduce contamination with normal oropharyngeal flora. 2) Encourage deep cough with expectoration of the sputum into a sterile specimen collection cup that is labeled with the patient's name and second patient identifier. 3) Do not send saliva (spit) for culture 110

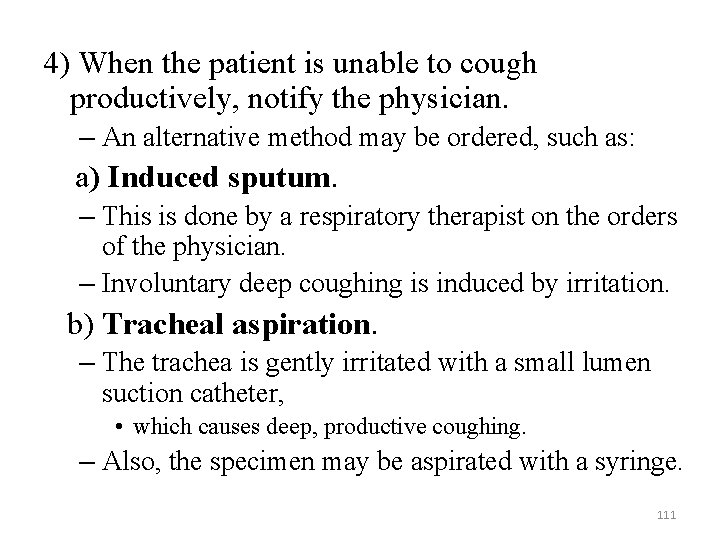
4) When the patient is unable to cough productively, notify the physician. – An alternative method may be ordered, such as: a) Induced sputum. – This is done by a respiratory therapist on the orders of the physician. – Involuntary deep coughing is induced by irritation. b) Tracheal aspiration. – The trachea is gently irritated with a small lumen suction catheter, • which causes deep, productive coughing. – Also, the specimen may be aspirated with a syringe. 111

c) Bronchial washings. – These are done by the physician in the operating room at the time of bronchoscopic examination. 5) A small amount of sputum is all that is required, but it must be sputum and not oral secretions. 112

Specimens of Wound Exudate. • Follow these steps for using a sterile transport swab in collecting wound exudate specimens. 1) Gently cleanse the area, using dry, sterile gauze to remove any contaminants. 2) Using a sterile red-stopper swab culture collection system, introduce deeply enough to obtain a moist specimen; replace the swab in the container. • Do not break the container. • 3) Store at room temperature. 113

Stool for Culture. When collecting stool specimens, follow these guidelines. 1) A small amount is all that is required, about the size of a walnut. – If several different types of cultures are requested, submit a walnut-sized sample for each. – Place the specimen in transport medium or in a sterile leakproof container. 2) When stool cultures are not readily obtainable, rectal swabs are acceptable; – however, it must be indicated whether the specimen is a stool or a rectal swab. 114

Use of Sterile Swab (Red-Stopper) Collection Kit • The swab system is guaranteed sterile until the seal is broken. • Directions for use: 1) Peel open and remove the swab from the package. 2) Remove the cap/swab stick from the tube. 3) Collect the appropriate specimen and put the cap/swab into the tube. Push the cap to bring the swab into contact with the transport medium. 4) Print the patient's name, second patient identifier and the culture site on the specimen tube. 5) Place the specimen in a zip-lock bag and put the completed test request form in the side pouch 6) Store it at room temperature. 7) Send specimen to the laboratory 115

116

Stool Specimen Collection: Freshly passed stool samples • avoid specimens from a bed pan Use sterile or clean container • do not clean with disinfectant During an outbreak - collect from 10 -20 patients 117

Stool Specimen Analysis of fecal material can detect pathological conditions ie: tumors, haemorrhage, infection • Tests – Occult blood – Pus – Ova & Parasites 118

• ? Chemical preservatives • Medical aseptic technique • To lab on time • Labelling • Documentation Guaiac Test Colorectal cancer screening test FOBT Hemoccult slide test 119

• Color – melena • • • Odor Consistency Frequency Amount Shape Constituents 120

Guaiac Test • Single positive test result does not confirm bleeding or colorectal cancer. • Repeat test 3 X • Meat free, high residue diet 121

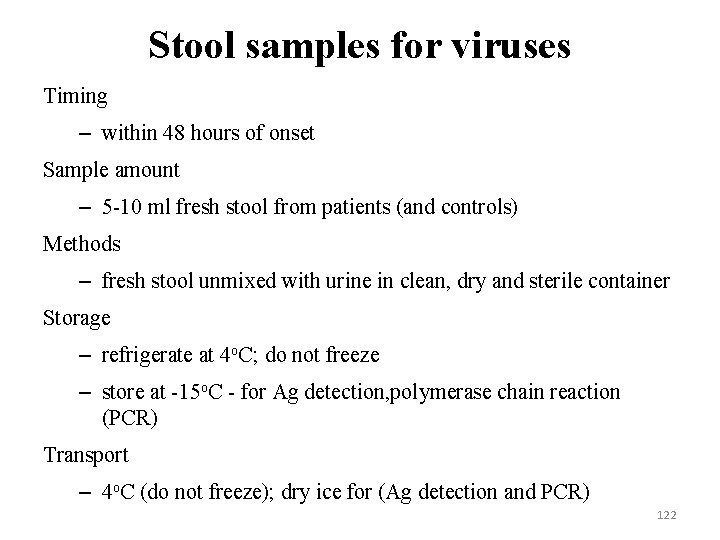
Stool samples for viruses Timing – within 48 hours of onset Sample amount – 5 -10 ml fresh stool from patients (and controls) Methods – fresh stool unmixed with urine in clean, dry and sterile container Storage – refrigerate at 4 o. C; do not freeze – store at -15 o. C - for Ag detection, polymerase chain reaction (PCR) Transport – 4 o. C (do not freeze); dry ice for (Ag detection and PCR) 122

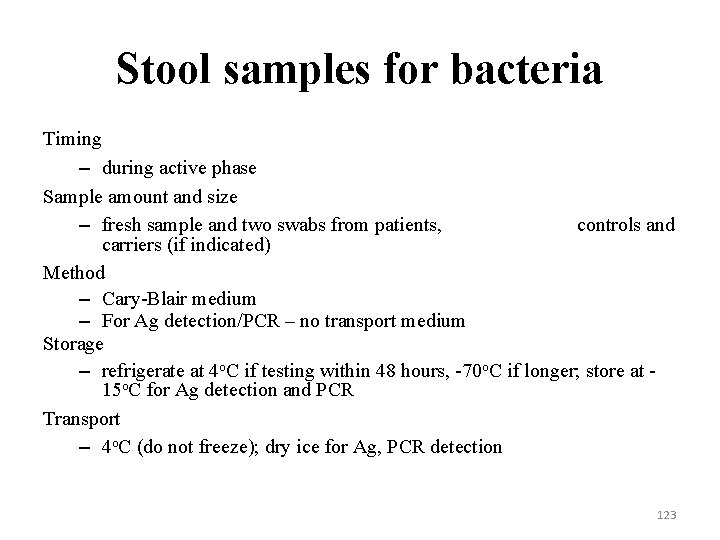
Stool samples for bacteria Timing – during active phase Sample amount and size – fresh sample and two swabs from patients, controls and carriers (if indicated) Method – Cary-Blair medium – For Ag detection/PCR – no transport medium Storage – refrigerate at 4 o. C if testing within 48 hours, -70 o. C if longer; store at 15 o. C for Ag detection and PCR Transport – 4 o. C (do not freeze); dry ice for Ag, PCR detection 123

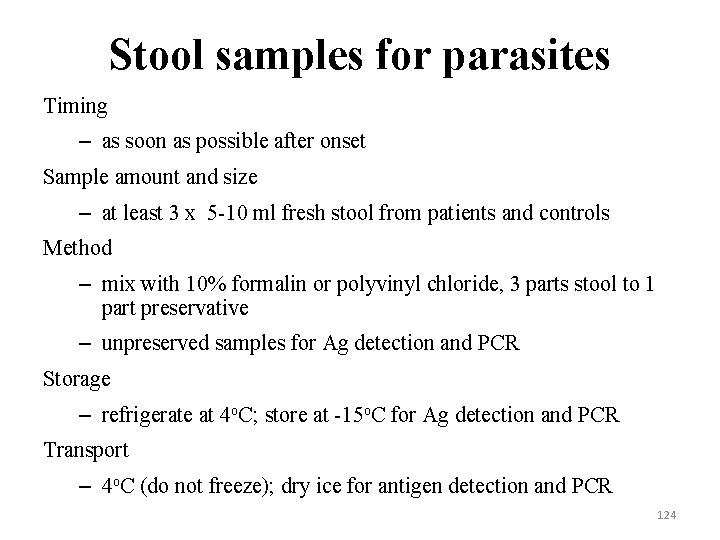
Stool samples for parasites Timing – as soon as possible after onset Sample amount and size – at least 3 x 5 -10 ml fresh stool from patients and controls Method – mix with 10% formalin or polyvinyl chloride, 3 parts stool to 1 part preservative – unpreserved samples for Ag detection and PCR Storage – refrigerate at 4 o. C; store at -15 o. C for Ag detection and PCR Transport – 4 o. C (do not freeze); dry ice for antigen detection and PCR 124

Rectal swabs Advantage – convenient – adapted to small children, debilitated patients and other situations where voided stool sample not feasible Drawbacks – no macroscopic assessment possible – less material available – not recommended for viruses 125

Post-mortem samples Collection Biopsy relevant tissues • place in formalin for histopathology • place in transport medium for microbiological testing • place in sterile saline for isolation of viral pathogens 126

Handling and transportation Fixed specimens can be transported at ambient temperatures • transport specimens in transport media within 24 h at ambient temperature • transport specimens in sterile saline at 4 -8 o. C within 48 h 127

Biosafety: protect the patient Use single use equipment Disinfect Work in a clean, dedicated area 128

Biosafety: protect yourself Use personal protective equipment – disposable gloves – laboratory coats / gown – mask – protective eyewear / face shields if procedure is likely to generate aerosols If no sharps container: collect sharps immediately to prevent needlestick injury Have first aid kit readily accessible Do not reuse contaminated equipment 129

Biosafety: protect others, the environment Package samples appropriately for transport Decontaminate spills - 10% bleach after wiping the surface clean Disinfect working areas for future use - 1% household bleach daily Soak contaminated non-disposable equipment/material in 1% household bleach for 5 minutes – wash in soapy water before re-use, sterilize if necessary Place waste in leak-proof biohazard bags - ensure safe final management of waste Protect cleaning/decontamination personnel with protective coat, thick rubber gloves 130

Cerebrospinal fluid (CSF) Collection Lumbar puncture Sterile tubes Aseptic conditions Trained person 131

Handling and transportation Bacteria • preferably in trans-isolate medium, • pre-warmed to 25 -37°C before inoculation OR • transport at ambient temperature (relevant pathogens do not survive at low temperatures) Viruses • transport at 4 -8 o. C (if up to 48 hrs or -70 o. C for longer duration) 132
 Clinical pathology accreditation
Clinical pathology accreditation Clinical pathology meaning
Clinical pathology meaning Clinical pathology accreditation
Clinical pathology accreditation Nabl assessor course
Nabl assessor course Normal alt in cats
Normal alt in cats Clinical pathology accreditation
Clinical pathology accreditation Hệ hô hấp
Hệ hô hấp ưu thế lai là gì
ưu thế lai là gì Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Tư thế ngồi viết
Tư thế ngồi viết Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Tư thế ngồi viết
Tư thế ngồi viết Giọng cùng tên là
Giọng cùng tên là Chó sói
Chó sói Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Frameset trong html5
Frameset trong html5 Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau 101012 bằng
101012 bằng Lời thề hippocrates
Lời thề hippocrates Thang điểm glasgow
Thang điểm glasgow đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tính thế năng
Công thức tính thế năng Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Hát lên người ơi alleluia
Hát lên người ơi alleluia điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Các số nguyên tố là gì
Các số nguyên tố là gì Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Tia chieu sa te
Tia chieu sa te đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Fish and shellfish pathology
Fish and shellfish pathology Anatomical pathology definition
Anatomical pathology definition Methods of pathology
Methods of pathology Cryptology and pathology
Cryptology and pathology Desematocele
Desematocele Driven snow appearance meaning
Driven snow appearance meaning Plant pathology
Plant pathology Digital pathology
Digital pathology Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download What pathology
What pathology Female reproductive system pathology
Female reproductive system pathology Body fluid
Body fluid Gross pathology
Gross pathology Utah web path
Utah web path Urinary bladder carcinoma
Urinary bladder carcinoma Pathology outline
Pathology outline Oral pathology
Oral pathology E3bp speech pathology
E3bp speech pathology Oral pathology
Oral pathology Plant pathology
Plant pathology Define seed pathology
Define seed pathology Xanthelasma pathology
Xanthelasma pathology Forensic pathology definition
Forensic pathology definition Bicep
Bicep Plant pathology
Plant pathology Pancreatitis film
Pancreatitis film Female reproductive system pathology
Female reproductive system pathology Heart
Heart Asthmatic lung x ray
Asthmatic lung x ray Cpc lab test
Cpc lab test Quality management in anatomic pathology
Quality management in anatomic pathology Lines of zahn gross
Lines of zahn gross Gross pathology definition
Gross pathology definition Barrel chest with emphysema
Barrel chest with emphysema Nutmeg liver slideshare
Nutmeg liver slideshare Sample diagnostic report for speech-language pathology
Sample diagnostic report for speech-language pathology Axis wheel ecg
Axis wheel ecg Robbins basic pathology
Robbins basic pathology Pathology
Pathology Pathology
Pathology Cryptology and pathology
Cryptology and pathology Thyroid pathology
Thyroid pathology Data-centric pathology
Data-centric pathology Forensic pathology quiz
Forensic pathology quiz National pathology week
National pathology week Female reproductive system pathology
Female reproductive system pathology Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Highest clouds
Highest clouds Leeds pathology tests and tubes
Leeds pathology tests and tubes Social pathology
Social pathology University of leeds virtual pathology
University of leeds virtual pathology Marcroft grains pathology
Marcroft grains pathology Plant pathology
Plant pathology Pathology
Pathology History of fungicides
History of fungicides Banff pathology course
Banff pathology course Bcc pathology
Bcc pathology Epath pathology
Epath pathology Female reproductive system pathology
Female reproductive system pathology Plant pathology
Plant pathology Crenation
Crenation Archie buskin
Archie buskin Mucoepidermoid carcinoma histology
Mucoepidermoid carcinoma histology Giant cell tumour
Giant cell tumour Emr pathology
Emr pathology Additive pathology
Additive pathology Raphe palatina
Raphe palatina Nanometer to millimeter
Nanometer to millimeter Pathology
Pathology Frcpath part 1
Frcpath part 1 Otorhinolaryngology combining form
Otorhinolaryngology combining form Pyelonephritis
Pyelonephritis Plant pathology
Plant pathology Wally langdon
Wally langdon Pathology
Pathology Echinococcus granulosus sketchy
Echinococcus granulosus sketchy Disease cycle
Disease cycle Folha heterofilia
Folha heterofilia Pathology conference
Pathology conference Thyroid pathology
Thyroid pathology Concomitant pathology
Concomitant pathology Introduction and importance of seed pathology
Introduction and importance of seed pathology Uterine seal
Uterine seal Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Chapter 17 oral pathology short answer questions
Chapter 17 oral pathology short answer questions