Clinical Dilemma Which Adjuvant Chemotherapy is Just Right



























- Slides: 58

Clinical Dilemma: Which Adjuvant Chemotherapy is Just Right? Dr. Maureen Trudeau Head, Division of Medical Oncology/Hematology Toronto Sunnybrook Regional Cancer Centre Associate Professor, University of Toronto June 15, 2007

Systemic Therapy - Chemotherapy • Overall survival improvement in clinical trials both for standard and newer treatments • Choice – for patients, for physicians (anthracycline +/- taxanes) • Better decision making aids – www. adjuvantonline. com • Molecular profiles – Oncotype Dx, Mammo. Print • Improved supportive care

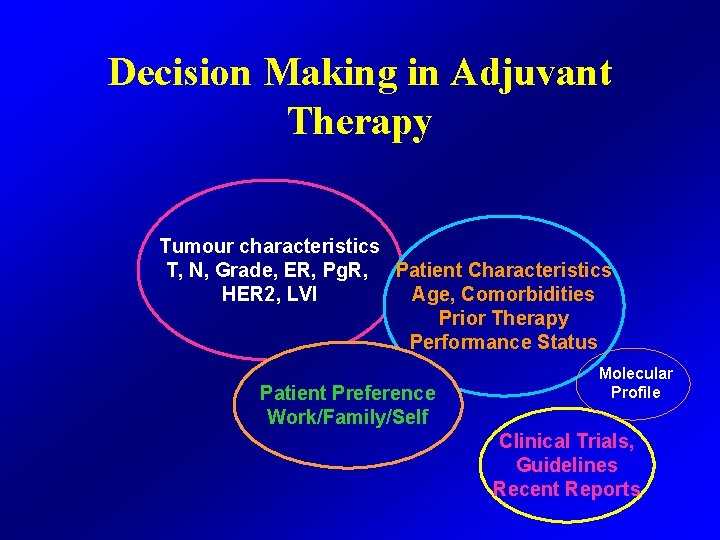
Decision Making in Adjuvant Therapy Tumour characteristics T, N, Grade, ER, Pg. R, Patient Characteristics Age, Comorbidities HER 2, LVI Prior Therapy Performance Status Patient Preference Work/Family/Self Molecular Profile Clinical Trials, Guidelines Recent Reports

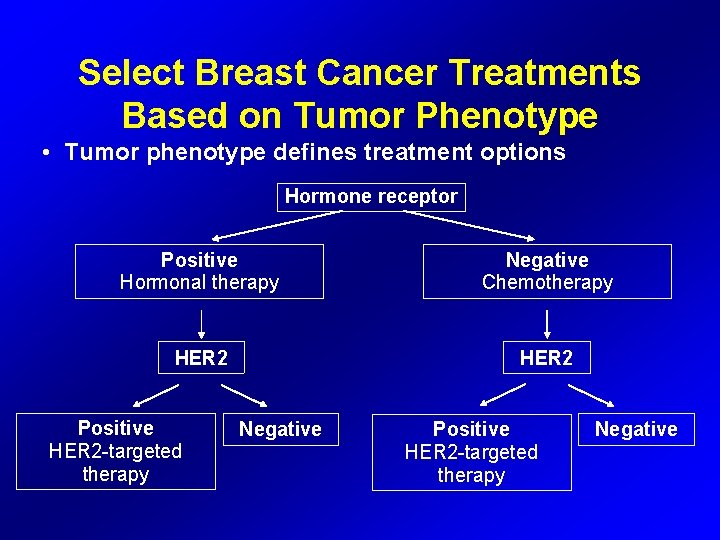
Select Breast Cancer Treatments Based on Tumor Phenotype • Tumor phenotype defines treatment options Hormone receptor Positive Hormonal therapy Negative Chemotherapy HER 2 Positive HER 2 -targeted therapy Negative


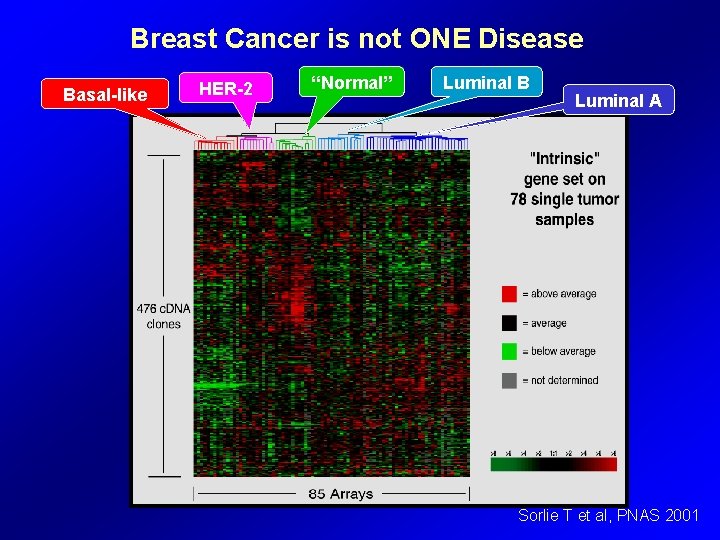
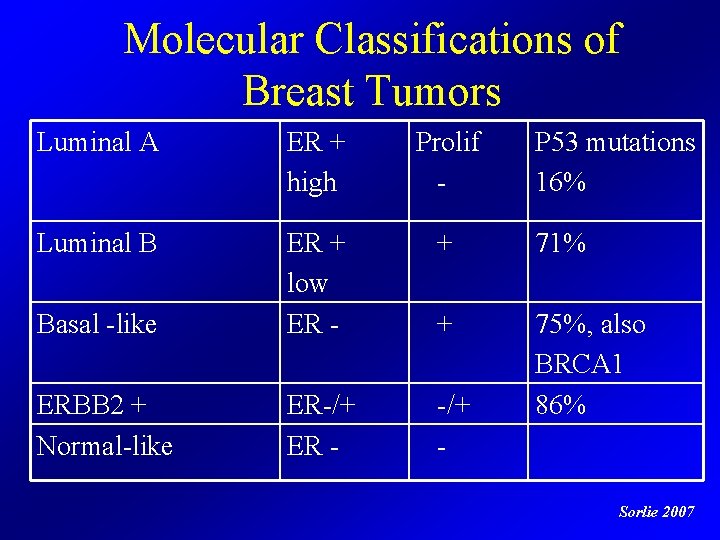
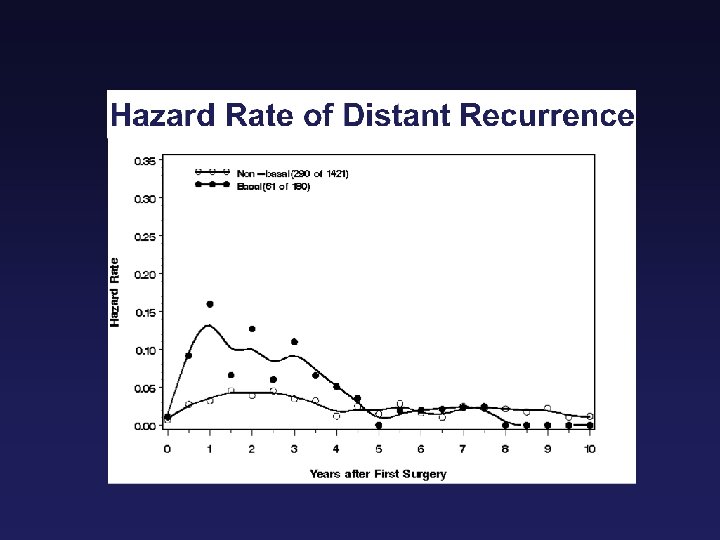
Breast Cancer is not ONE Disease Basal-like HER-2 “Normal” Luminal B Luminal A Sorlie T et al, PNAS 2001


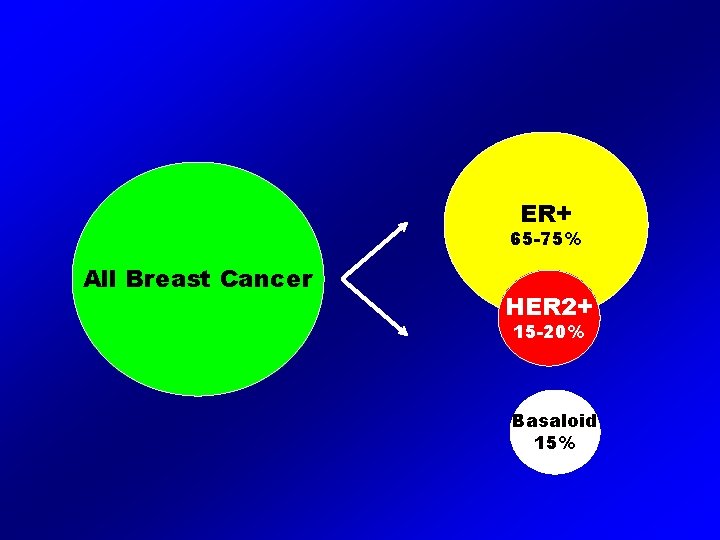
ER+ 65 -75% All Breast Cancer HER 2+ 15 -20% Basaloid 15%

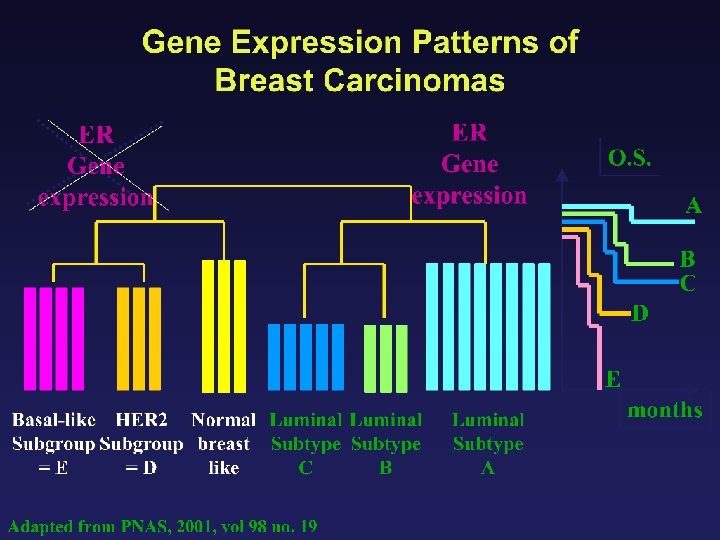
Molecular Classifications of Breast Tumors Luminal A ER + high Prolif - P 53 mutations 16% Luminal B + 71% Basal -like ER + low ER - + ERBB 2 + Normal-like ER-/+ ER - -/+ - 75%, also BRCA 1 86% Sorlie 2007








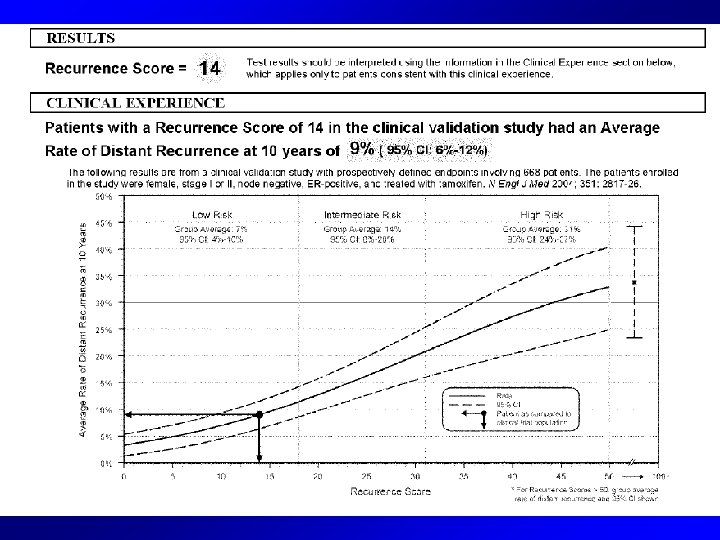
Oncotype DX • A multigene assay to predict recurrence of Tamoxifen-treated, node-negative breast cancer (Paik NEJM 204) • 21 genes - proliferation (5), invasion (2), HER 2 (2), Estrogen (4) , 3 others and 5 reference genes with a Recurrence Score (RS) algorithm • For node negative, tam treated (JCO 2007): –Luminal A = low risk oncotype DX –Luminal B = mod/high risk







Adapted by Dr. Maureen E. Trudeau, MD

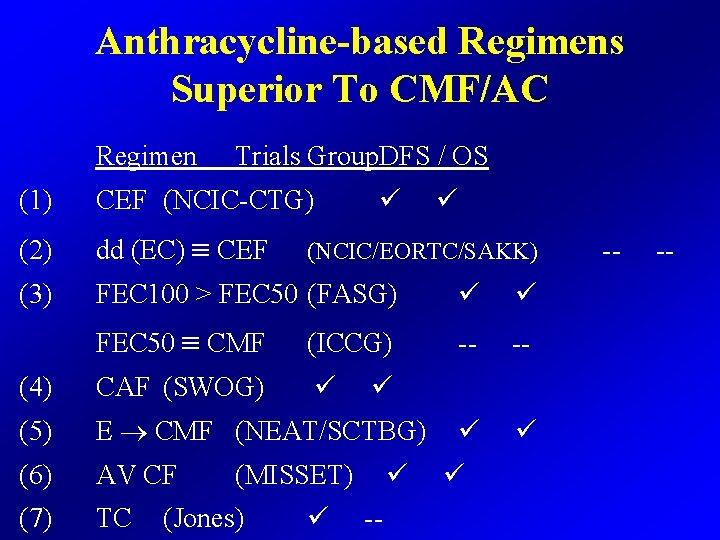
Anthracycline-based Regimens Superior To CMF/AC Regimen Trials Group. DFS / OS (1) CEF (NCIC-CTG) (2) dd (EC) CEF (3) FEC 100 > FEC 50 (FASG) FEC 50 CMF (ICCG) -- -- (4) CAF (SWOG) (5) E CMF (NEAT/SCTBG) (6) AV CF (7) TC (NCIC/EORTC/SAKK) (MISSET) (Jones) -- -- --

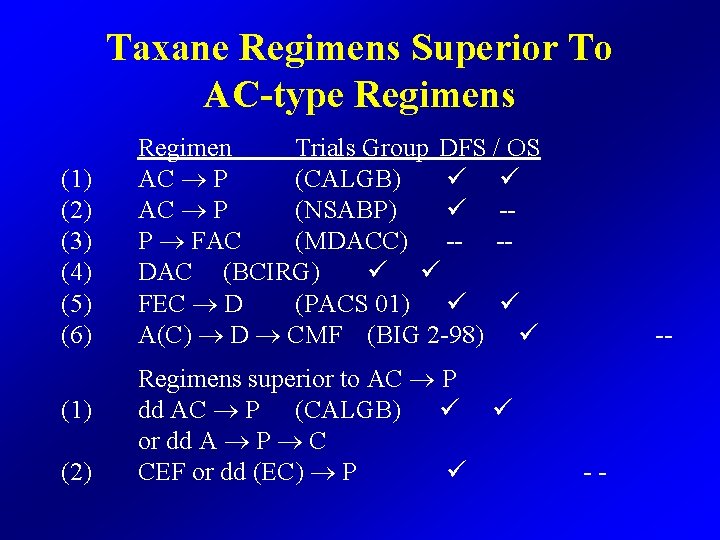
Taxane Regimens Superior To AC-type Regimens (1) (2) (3) (4) (5) (6) (1) (2) Regimen Trials Group DFS / OS AC P (CALGB) AC P (NSABP) -P FAC (MDACC) -- -DAC (BCIRG) FEC D (PACS 01) A(C) D CMF (BIG 2 -98) Regimens superior to AC P dd AC P (CALGB) or dd A P C CEF or dd (EC) P --

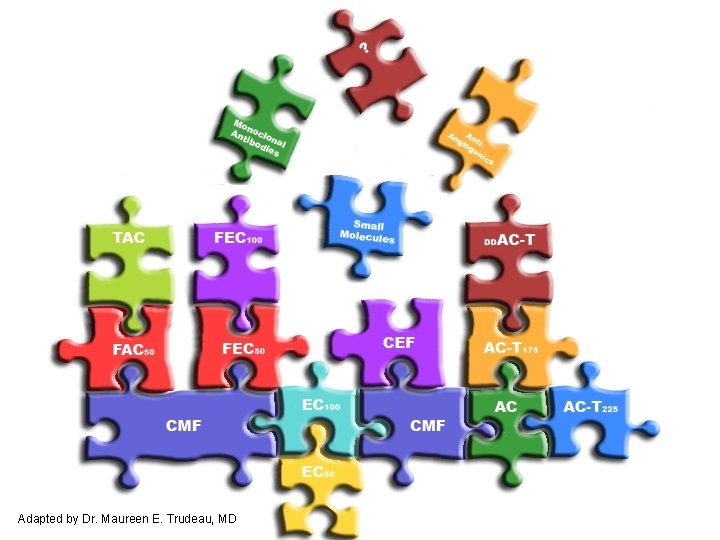
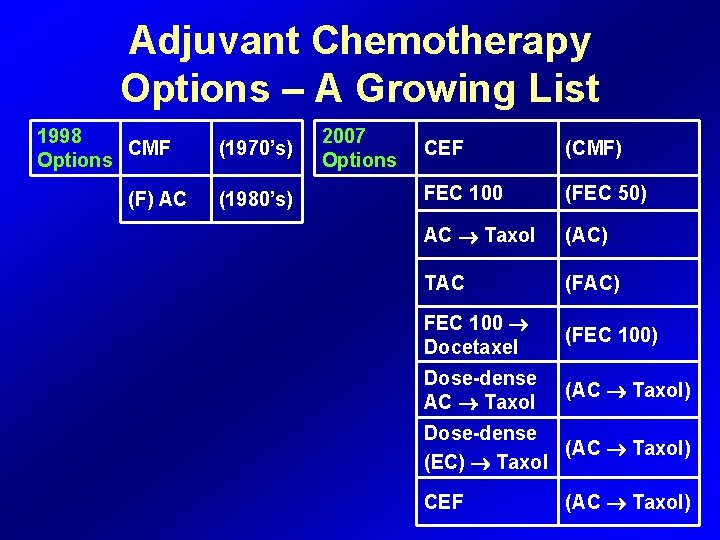
Adjuvant Chemotherapy Options – A Growing List 1998 CMF Options (F) AC (1970’s) (1980’s) 2007 Options CEF (CMF) FEC 100 (FEC 50) AC Taxol (AC) TAC (FAC) FEC 100 Docetaxel (FEC 100) Dose-dense AC Taxol (AC Taxol) Dose-dense (AC Taxol) (EC) Taxol CEF (AC Taxol)

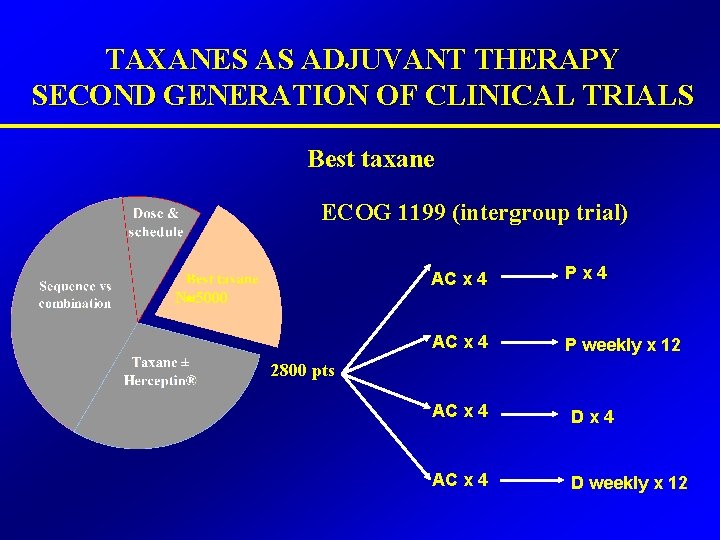
TAXANES AS ADJUVANT THERAPY SECOND GENERATION OF CLINICAL TRIALS Best taxane ECOG 1199 (intergroup trial) N 5000 AC x 4 Px 4 AC x 4 P weekly x 12 2800 pts AC x 4 Dx 4 AC x 4 D weekly x 12

Are There Factors that May Predict Response or Suggest which Therapy to Use?

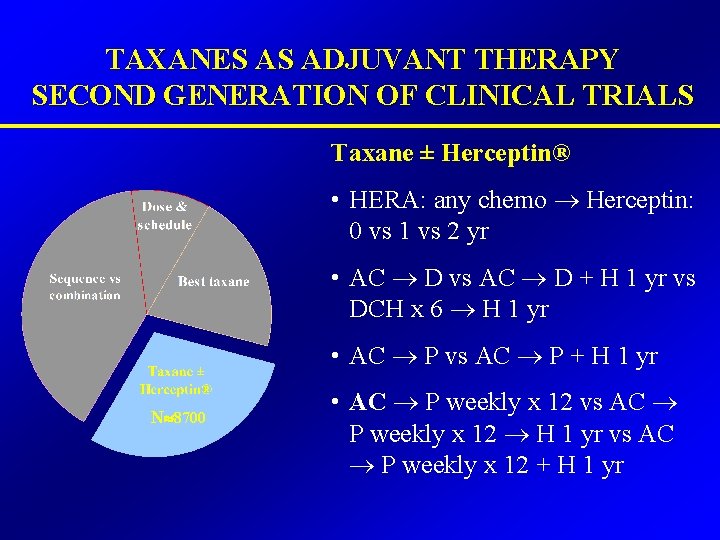
TAXANES AS ADJUVANT THERAPY SECOND GENERATION OF CLINICAL TRIALS Taxane ± Herceptin® • HERA: any chemo Herceptin: 0 vs 1 vs 2 yr • AC D vs AC D + H 1 yr vs DCH x 6 H 1 yr • AC P vs AC P + H 1 yr N 8700 • AC P weekly x 12 vs AC P weekly x 12 H 1 yr vs AC P weekly x 12 + H 1 yr

Trastuzumab DFS Median follow-up HERA 1 year Combined analysis 2 years DH 2 years BCIRG 006 DCarbo. H 2 years BCIRG 006 AC Fin. HER VH / DH 3 years CEF 0 Favors Trastuzumab 1 Favors no 2 Trastuzumab HR Piccart-Gebhart et al 2005; Romond et al 2005; Slamon et al 2005; Joensuu et al 2005

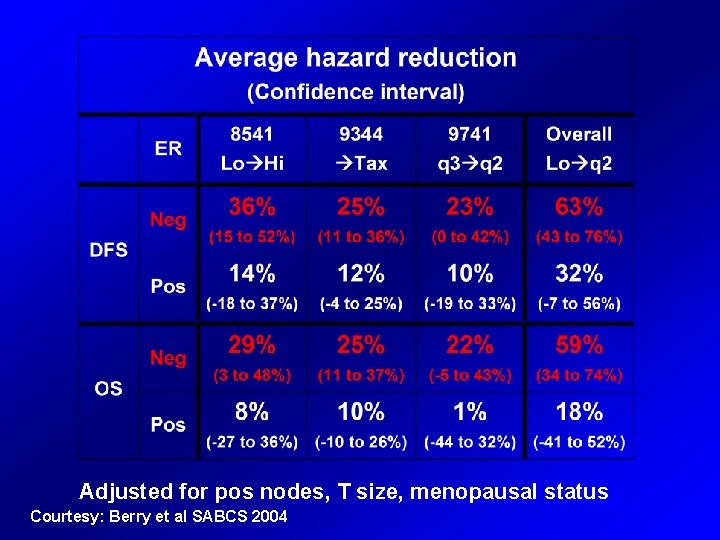
Adjusted for pos nodes, T size, menopausal status Courtesy: Berry et al SABCS 2004

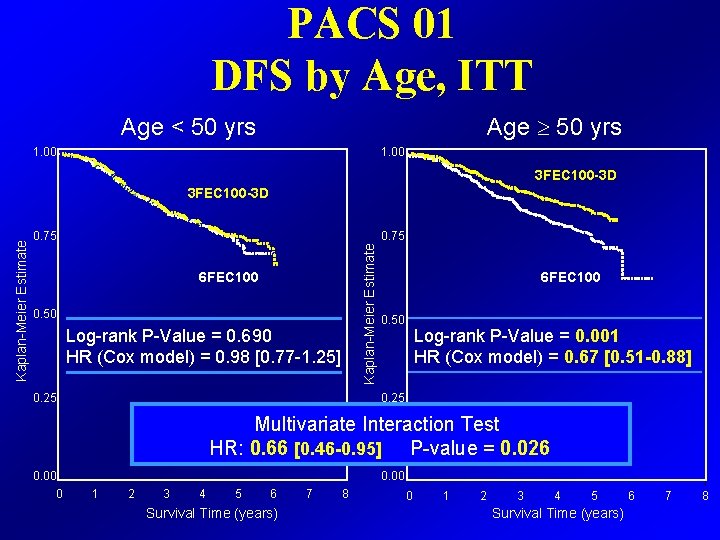
PACS 01 DFS by Age, ITT Age 50 yrs Age < 50 yrs 1. 00 3 FEC 100 -3 D 0. 75 Kaplan-Meier Estimate 3 FEC 100 -3 D 6 FEC 100 0. 50 Log-rank P-Value = 0. 690 HR (Cox model) = 0. 98 [0. 77 -1. 25] 0. 25 6 FEC 100 0. 50 Log-rank P-Value = 0. 001 HR (Cox model) = 0. 67 [0. 51 -0. 88] 0. 25 Multivariate Interaction Test HR: 0. 66 [0. 46 -0. 95] P-value = 0. 026 0. 00 0 1 2 3 4 5 6 Survival Time (years) 7 8 0 1 2 3 4 5 Survival Time (years) 6 7 8

TAC vs FAC DFS by HER 2 Status (Centrally reviewed, FISH centrally reviewed) % Alive and Disease-Free 100 Negative 90 100 Positive 90 80 TAC FAC 70 60 HR = 0. 76 P = 0. 046 50 70 60 HR = 0. 60 P = 0. 0088 FAC 50 0 6 12 18 24 30 36 42 48 54 60 66 Time to First Event Ratio of HRs 0. 85 p= 0. 4122 NEJM 2005

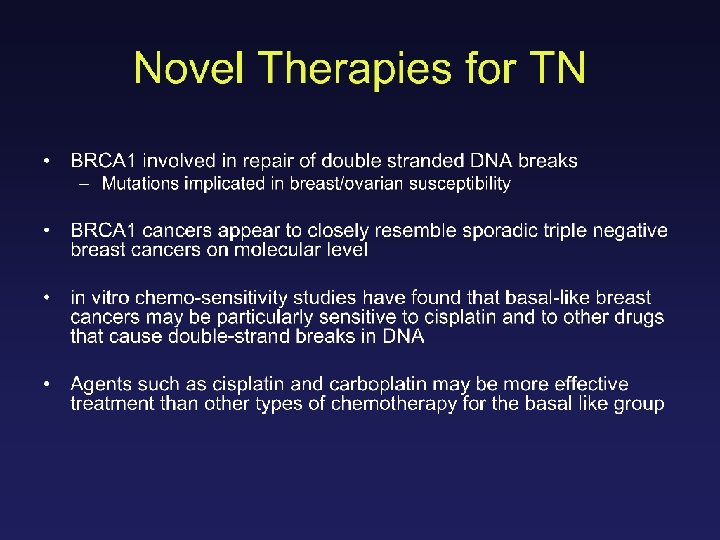
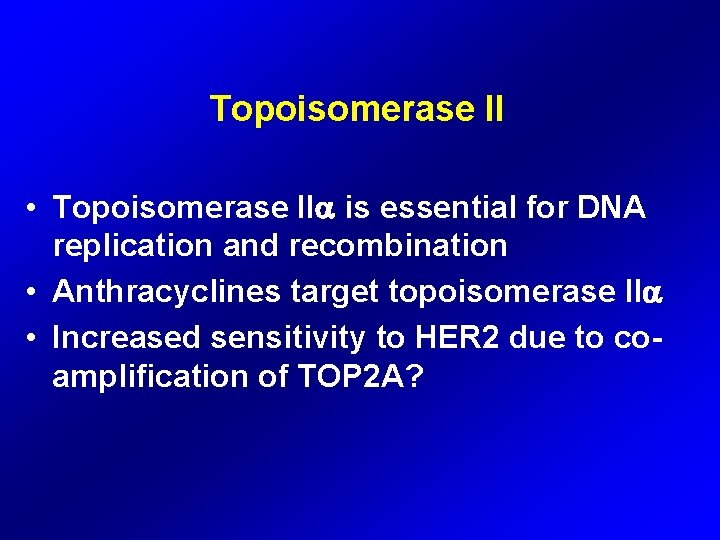
Topoisomerase II • Topoisomerase II is essential for DNA replication and recombination • Anthracyclines target topoisomerase II • Increased sensitivity to HER 2 due to coamplification of TOP 2 A?

A pooled analysis on the interaction between HER-2 expression and responsiveness of breast cancer to adjuvant chemotherapy Alessandra Gennari, Maria Pia Sormani, Matteo Puntoni and Paolo Bruzzi National Cancer Research Institute - Genoa and University of Genoa - Italy SABCS 2006

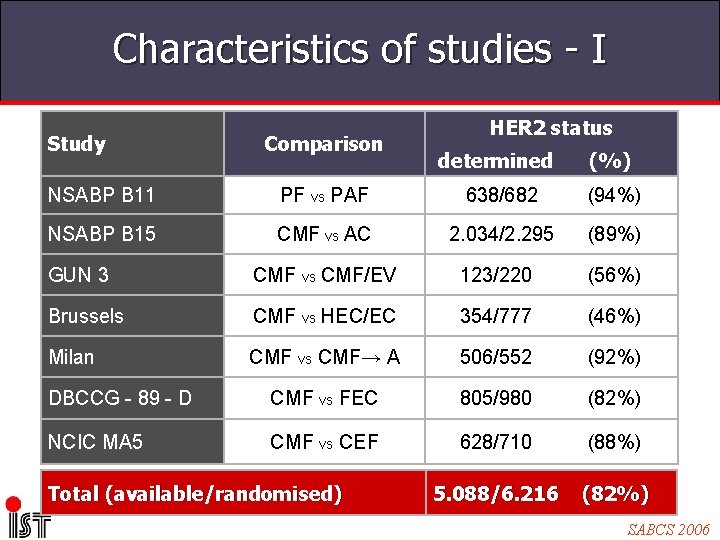
Characteristics of studies - I Study Comparison HER 2 status determined (%) NSABP B 11 PF vs PAF 638/682 (94%) NSABP B 15 CMF vs AC 2. 034/2. 295 (89%) GUN 3 CMF vs CMF/EV 123/220 (56%) Brussels CMF vs HEC/EC 354/777 (46%) Milan CMF vs CMF→ A 506/552 (92%) DBCCG - 89 - D CMF vs FEC 805/980 (82%) NCIC MA 5 CMF vs CEF 628/710 (88%) 5. 088/6. 216 (82%) Total (available/randomised) SABCS 2006

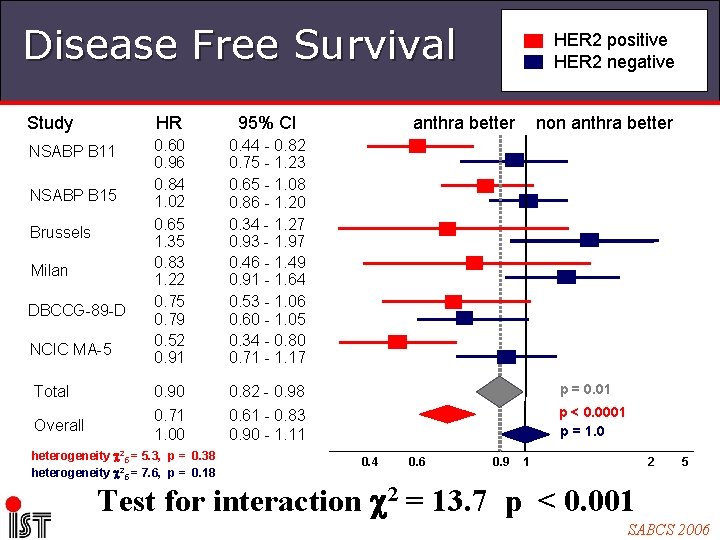
Disease Free Survival HER 2 positive HER 2 negative Study HR 95% CI NSABP B 11 0. 60 0. 96 0. 84 1. 02 0. 65 1. 35 0. 83 1. 22 0. 75 0. 79 0. 52 0. 91 0. 44 - 0. 82 0. 75 - 1. 23 0. 65 - 1. 08 0. 86 - 1. 20 0. 34 - 1. 27 0. 93 - 1. 97 0. 46 - 1. 49 0. 91 - 1. 64 0. 53 - 1. 06 0. 60 - 1. 05 0. 34 - 0. 80 0. 71 - 1. 17 Total 0. 90 0. 82 - 0. 98 p = 0. 01 Overall 0. 71 1. 00 0. 61 - 0. 83 0. 90 - 1. 11 p < 0. 0001 p = 1. 0 NSABP B 15 Brussels Milan DBCCG-89 -D NCIC MA-5 heterogeneity 25 = 5. 3, p = 0. 38 heterogeneity 25 = 7. 6, p = 0. 18 anthra better 0. 4 0. 6 0. 9 non anthra better 2 1 5 Test for interaction 2 = 13. 7 p < 0. 001 SABCS 2006

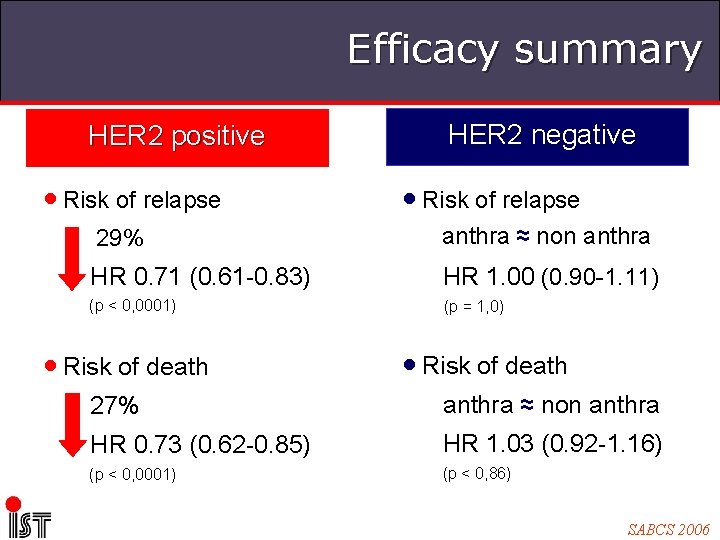
Efficacy summary HER 2 positive • Risk of relapse HER 2 negative • Risk of relapse 29% anthra ≈ non anthra HR 0. 71 (0. 61 -0. 83) HR 1. 00 (0. 90 -1. 11) (p < 0, 0001) (p = 1, 0) • Risk of death 27% anthra ≈ non anthra HR 0. 73 (0. 62 -0. 85) HR 1. 03 (0. 92 -1. 16) (p < 0, 0001) (p < 0, 86) SABCS 2006

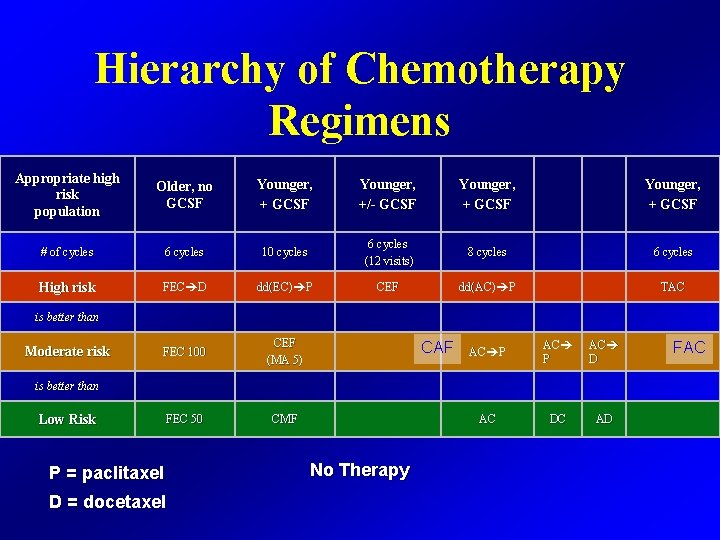
Hierarchy of Chemotherapy Regimens Appropriate high risk population Older, no GCSF Younger, +/- GCSF Younger, + GCSF # of cycles 6 cycles 10 cycles 6 cycles (12 visits) 8 cycles 6 cycles High risk FEC D dd(EC) P CEF dd(AC) P TAC FEC 100 CEF (MA 5) FEC 50 CMF is better than Moderate risk CAF AC AC P AC AC DC AD is better than Low Risk P = paclitaxel D = docetaxel No Therapy FAC

The choice of chemotherapy Depends on the following: • Tumour characteristics and risk of relapse • Patient comorbidities • Patient age • Social determinants • Drug availability / costs • Physician or patient preference

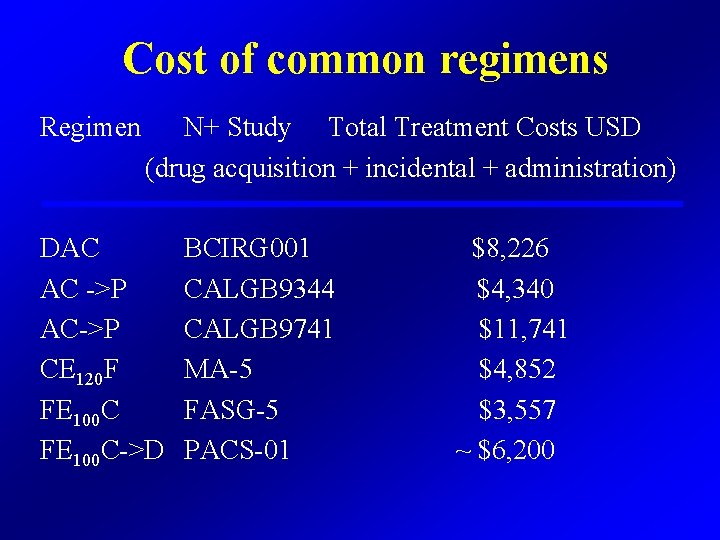
Cost of common regimens Regimen N+ Study Total Treatment Costs USD (drug acquisition + incidental + administration) DAC AC ->P AC->P CE 120 F FE 100 C->D BCIRG 001 CALGB 9344 CALGB 9741 MA-5 FASG-5 PACS-01 $8, 226 $4, 340 $11, 741 $4, 852 $3, 557 ~ $6, 200

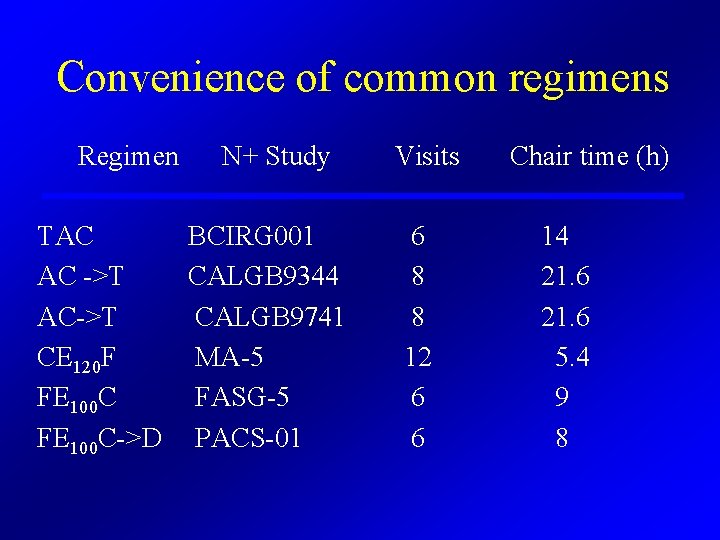
Convenience of common regimens Regimen N+ Study TAC BCIRG 001 AC ->T CALGB 9344 AC->T CALGB 9741 CE 120 F MA-5 FE 100 C FASG-5 FE 100 C->D PACS-01 Visits 6 8 8 12 6 6 Chair time (h) 14 21. 6 5. 4 9 8

Where are we going? Adapted by Dr. Maureen E. Trudeau, MD

Cases

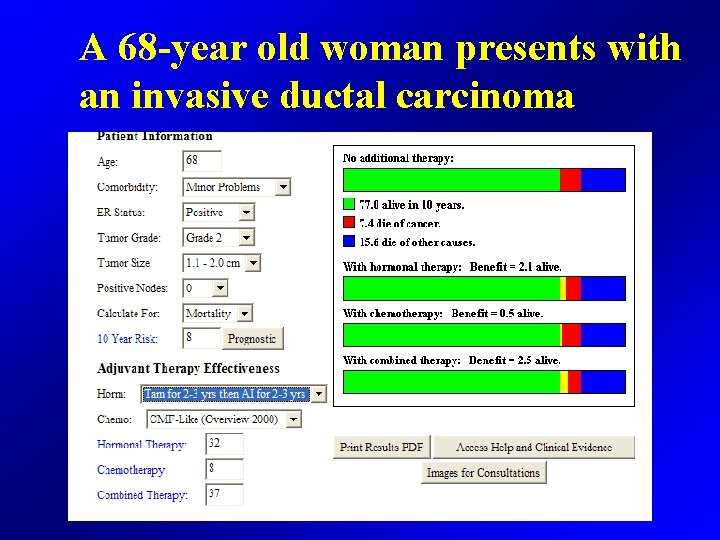
A 68 -year old woman presents with an infiltrating duct carcinoma • 1. 2 cm in size • ER 80% PR 60% • HER 2 • Sentinel node negative

A 68 -year old woman presents with an invasive ductal carcinoma

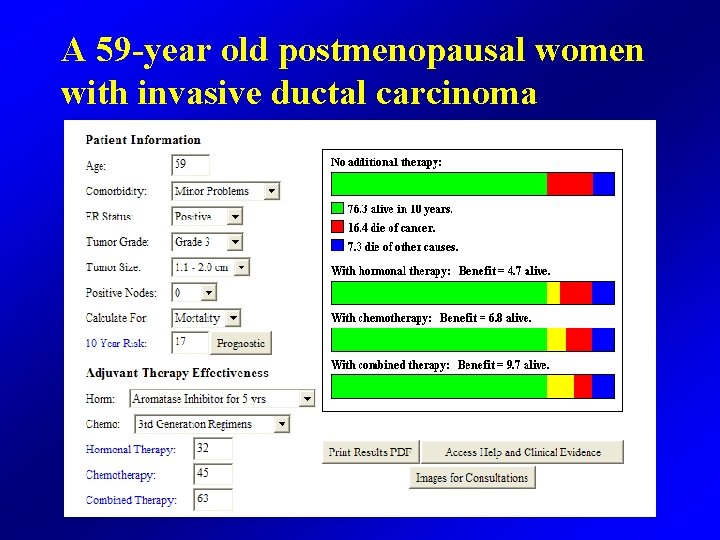
A 59 -year old postmenopausal woman with invasive ductal carcinoma • 1. 9 cm in size • ER 30% PR 0% • HER 2+ (3+ by IHC) • Grade 3 • Sentinel node negative

A 59 -year old postmenopausal women with invasive ductal carcinoma

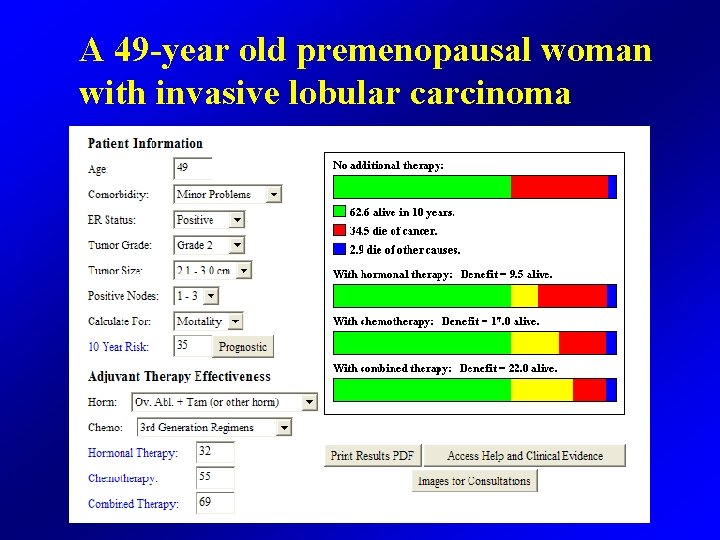
A 49 -year old premenopausal woman with invasive lobular carcinoma • 2. 5 cm in size • ER 70% PR 30% • HER 2 • Grade 2 • 2/10 positive lymph nodes

A 49 -year old premenopausal woman with invasive lobular carcinoma

A 39 -year old premenopausal woman with invasive ductal carcinoma • 2. 8 cm in size • ER 0% PR 0% • HER 2 • Grade 3 • 5 nodes positive


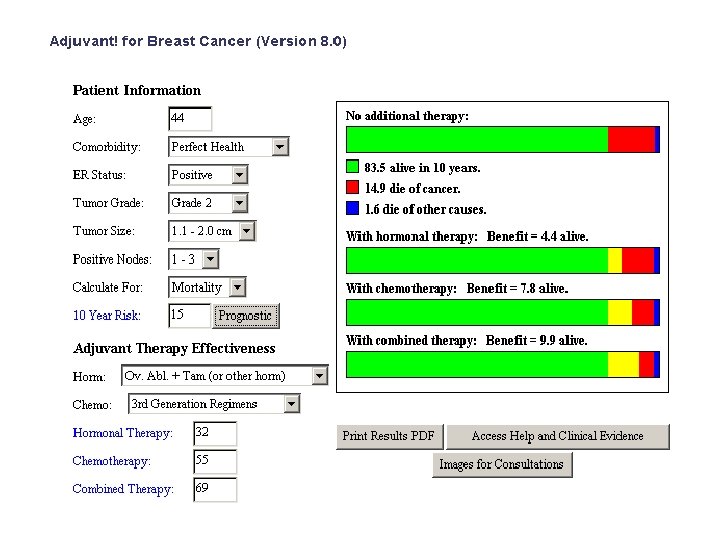
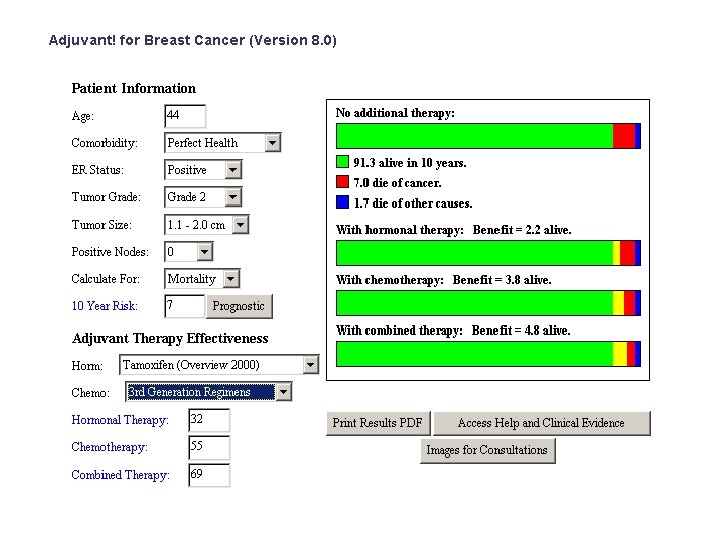
A 44 -year old premenopausal woman with invasive ductal carcinoma • 2. 0 cm in size • ER 100% PR 100% • HER 2 • Grade 2 • 1/17 nodes positive • 2 other smaller lesions, grade 1




 Right product right place right time right price
Right product right place right time right price Family time
Family time Gondor adjuvant
Gondor adjuvant Domenico galetta
Domenico galetta Adjuvant neoadjuvant palliative
Adjuvant neoadjuvant palliative Adjuvant nsclc
Adjuvant nsclc Bsa calculation formula for chemotherapy
Bsa calculation formula for chemotherapy Icd 9 code for oral thrush
Icd 9 code for oral thrush Principles of chemotherapy
Principles of chemotherapy General principles of chemotherapy
General principles of chemotherapy Chemotherapy
Chemotherapy Debra forman
Debra forman Parenting capacity assessment sa
Parenting capacity assessment sa 4ac 4t chemotherapy
4ac 4t chemotherapy The right man on the right place at the right time
The right man on the right place at the right time Who is the first person to arrive at juliet's tomb?
Who is the first person to arrive at juliet's tomb? Wanted a just right government cause and effect
Wanted a just right government cause and effect Wanted a just right government
Wanted a just right government Wanted a just right government cause and effect answer key
Wanted a just right government cause and effect answer key 3 bowls of porridge
3 bowls of porridge Just right state programme
Just right state programme Wanted a just right government
Wanted a just right government Not too big not too small just right
Not too big not too small just right Wanted a just right government cause and effect
Wanted a just right government cause and effect A just right government
A just right government Just right 1 esercizi svolti
Just right 1 esercizi svolti Left left right right go go go
Left left right right go go go You put your left foot in you put your left foot out
You put your left foot in you put your left foot out Left left right right go go go
Left left right right go go go Heinz dilemma
Heinz dilemma Vangin dilemma
Vangin dilemma Stag hunt vs prisoner's dilemma
Stag hunt vs prisoner's dilemma Sociological imagination abortion
Sociological imagination abortion Outcome of prisoner's dilemma
Outcome of prisoner's dilemma Fangens dilemma
Fangens dilemma Card stacking ad
Card stacking ad Prisoners dilemma
Prisoners dilemma Slack
Slack Global-local dilemma
Global-local dilemma Spt regel
Spt regel Dilemma del prigioniero spiegazione
Dilemma del prigioniero spiegazione Dialectici
Dialectici Prisoner's dilemma nash equilibrium
Prisoner's dilemma nash equilibrium Prisoners dilemma
Prisoners dilemma False dilemma in the crucible
False dilemma in the crucible Nærhetsetikk definisjon
Nærhetsetikk definisjon Perbedaan ethical dilemma dan ethical lapse
Perbedaan ethical dilemma dan ethical lapse Organizational dilemma in school
Organizational dilemma in school Argument from dilemma
Argument from dilemma Contoh red herring
Contoh red herring Personal moral dilemma
Personal moral dilemma Perbedaan ethical dilemma dan ethical lapse
Perbedaan ethical dilemma dan ethical lapse Management dilemma definition
Management dilemma definition False dichotomy
False dichotomy Constructive dilemma
Constructive dilemma Hva er etisk dilemma
Hva er etisk dilemma Euthyphro plato summary
Euthyphro plato summary Pooja morar
Pooja morar Dapps rule example
Dapps rule example