CLINICAL CHEMISTRY MLT 301 NONPROTEIN NITROGEN NPN LECTURE






- Slides: 18

CLINICAL CHEMISTRY (MLT 301) NONPROTEIN NITROGEN (NPN) LECTURE TWO Dr. Essam H. Jiffri 1

URIC ACID - Uric acid is the final breakdown product of purine metabolism. - Most mammals have the ability to catabolize purines one step further to allantoin, a much more water-soluble endproduct. - Purines such as adenosine and guanine, resulting from the breakdown of nucleic acids that are ingested or come from the destruction of tissue cells, are converted into uric acid, mainly in the liver. 2

URIC ACID - Uric acid is transported by the plasma from the liver to the kidney, where it is filtered by the glomerulus. - The uric acid in the glomerular filtrate, 98 % to 100% is reabsorbed in the proximal tubule, small amounts of uric acid are then secreted by the distal tubules and ultimately appear in the urine. 3

URIC ACID - The most important disease associated with elevated levels of uric acid in the plasma is gout. - Gout is a disease found primarily in males and usually first diagnosed between the ages of 3 O and 50. - Patients have pain and inflammation of the joints caused by precipitation of sodium urates in the joint resulting from the high levels of uric acid found in extracellular fluids 4

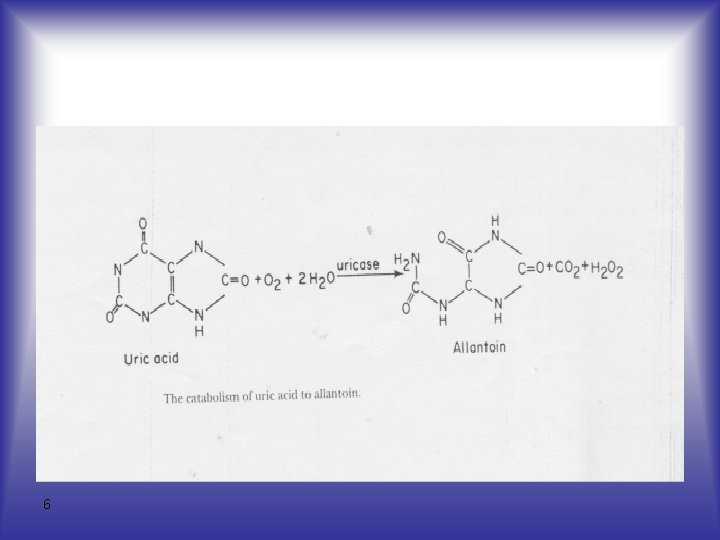
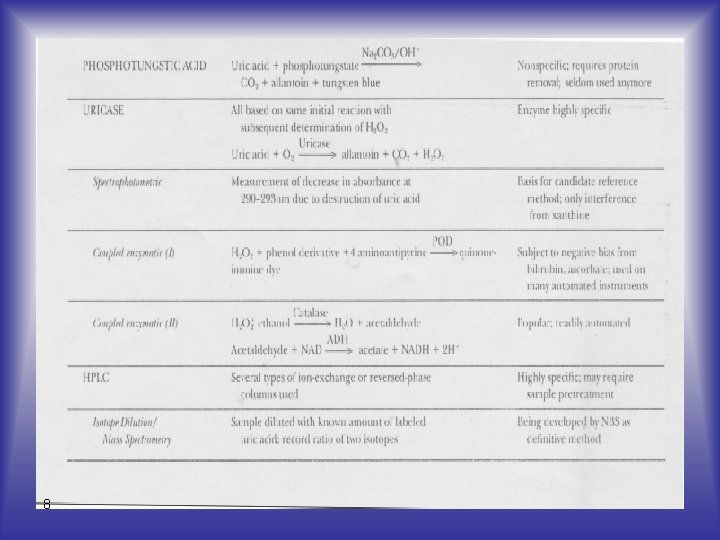
Analytical Methods - Uric acid is readily oxidized to allantoin and thus can function as a reducing agent in many reactions. 5

6

Analytical Methods - The most poplular method of this type is the Caraway method, which is based on the oxidation of the uric acid in a protein-free filtrate, with subsequent reduction of phosphotungstic acid to tungsten blue. 7

8

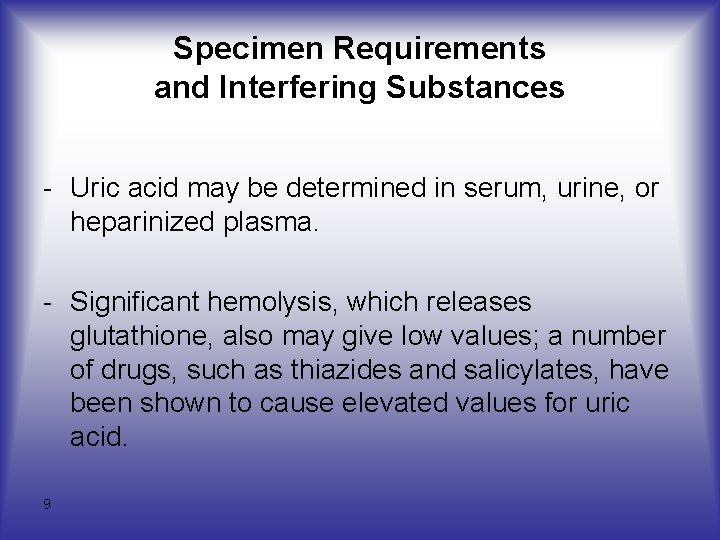
Specimen Requirements and Interfering Substances - Uric acid may be determined in serum, urine, or heparinized plasma. - Significant hemolysis, which releases glutathione, also may give low values; a number of drugs, such as thiazides and salicylates, have been shown to cause elevated values for uric acid. 9

Specimen Requirements and Interfering Substances - Diet in general may affect the uric acid levels, the patient need to be fasting. 10

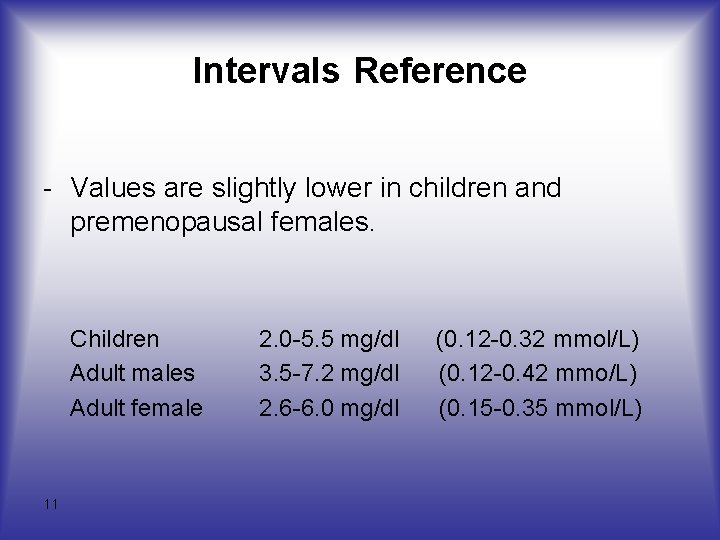
Intervals Reference - Values are slightly lower in children and premenopausal females. Children Adult males Adult female 11 2. 0 -5. 5 mg/dl 3. 5 -7. 2 mg/dl 2. 6 -6. 0 mg/dl (0. 12 -0. 32 mmol/L) (0. 12 -0. 42 mmo/L) (0. 15 -0. 35 mmol/L)

AMMONIA - The level of ammonia in the circulation is extremely low (11 -35 µmol/L). - It arises from the deamination of amino acids, which occurs mainly through the action of digestive and bacterial enzymes on proteins in the intestinal tract. 12

AMMONIA - Ammonia is also released from metabolic reactions that occur in skeletal muscle during exercise. - Severe liver disease represents the most common cause of disturbed ammonia metabolism. 13

Analytical Methods - There are two distinct approaches that have been used for the measurement of plasma ammonia. - One is a two-step approach in which ammonia is first isolated from the sample and then assayed. - The second involves direct measurement of ammonia by an enzymatic method or ionselective electrode. 14

15

Specimen Requirements and Interfering Substances - Ammonia levels rise rapidly in whole blood after drawing because of the deamination of amino acids. - EDTA is the preferred anticoagulant. 16

Specimen Requirements and Interfering Substances - Samples should be centrifuged at 0 to 40 C within 20 minutes of collection, and the plasma removed, and frozen plasma is reportedly stable for several days at -200 C. - Because red cells contain 2 to 3 times as much ammonia as plasma, hemolysis should be avoided. 17

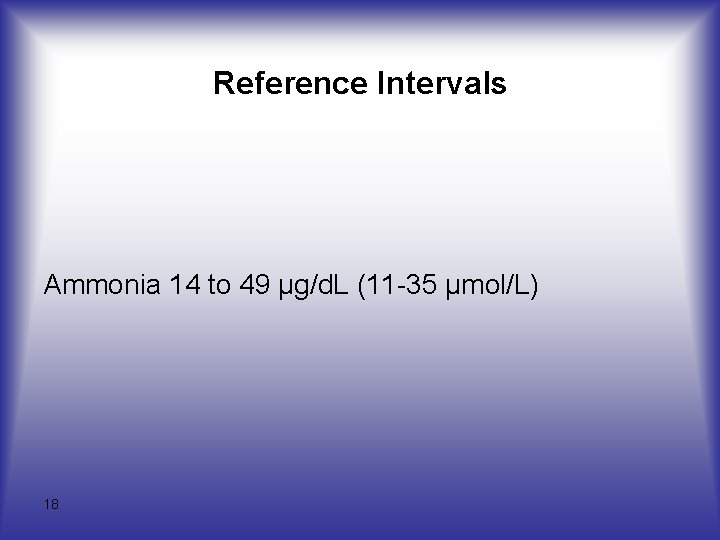
Reference Intervals Ammonia 14 to 49 µg/d. L (11 -35 µmol/L) 18
 Persenyawaan
Persenyawaan Mlt.moe.edu.sg.xuele
Mlt.moe.edu.sg.xuele Ligálás
Ligálás Npn chemistry
Npn chemistry 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Klb chemistry book 3 nitrogen and its compounds
Klb chemistry book 3 nitrogen and its compounds Ponto de saturação
Ponto de saturação Npn transistor doping
Npn transistor doping Npn
Npn Ic bjt
Ic bjt An npn transistor
An npn transistor Transistor
Transistor Non performing notes investing
Non performing notes investing Transistor fabrication techniques
Transistor fabrication techniques Npn
Npn Transistor sebagai saklar
Transistor sebagai saklar Dc analysis of bjt examples
Dc analysis of bjt examples Pnp symbol
Pnp symbol Site license health canada
Site license health canada