Clinical Case Scenarios Protocol References Section 7 Section


















- Slides: 28

Clinical Case Scenarios

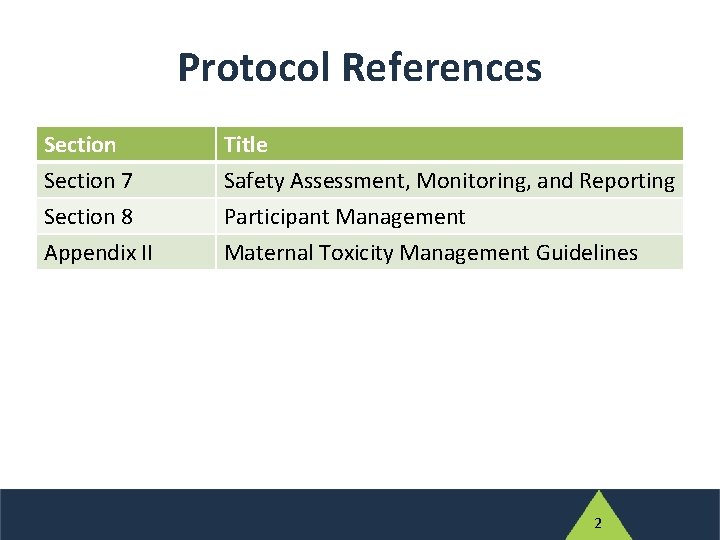
Protocol References Section 7 Section 8 Appendix II Title Safety Assessment, Monitoring, and Reporting Participant Management Maternal Toxicity Management Guidelines 2

Clinical Case Scenarios 3

Scenario 1 Consider a 20 year old pregnant woman who tested HIV-positive when presenting for antenatal care on 1 May 2018 and was then referred for possible participation in IMPAACT 2010. 4

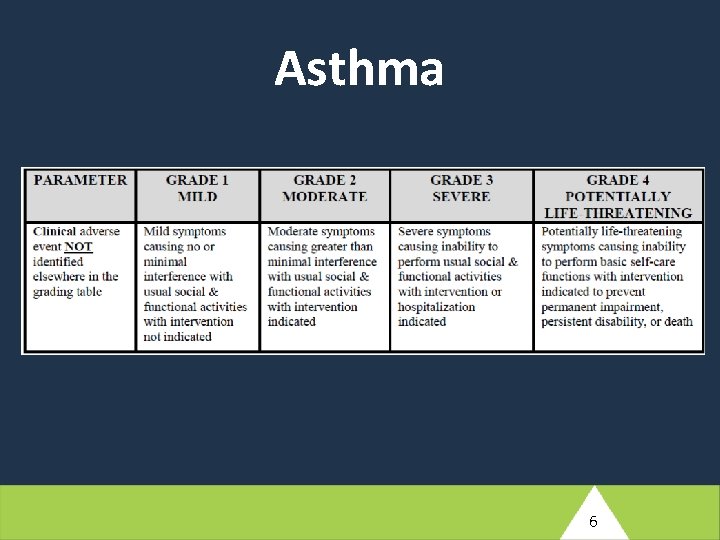
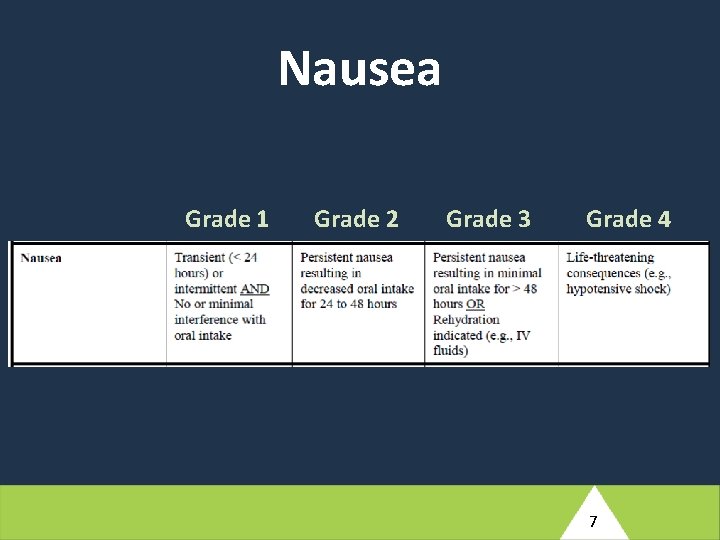
At screening for IMPAACT 2010, on 3 May 2018, this woman reports a history of asthma since childhood. She does not routinely take medication for this condition, but she has an inhaler that she uses when needed. She has had some nausea during the current pregnancy. This has been happening less often recently, but still happens from time to time. The nausea has not been accompanied by vomiting. Her screening laboratory results identify a grade 1 hemoglobin level; all other test results are normal. (1) Based on the brief description provided here, what additional information will the study clinician need to probe for to adequately source document this woman’s pre-existing medical conditions? 5

Asthma 6

Nausea Grade 1 Grade 2 Grade 3 Grade 4 7

At screening for IMPAACT 2010, on 3 May 2018, this woman reports a history of asthma since childhood. She does not routinely take medication for this condition, but she has an inhaler that she uses when needed. She has had some nausea during the current pregnancy. This has been happening less often recently, but still happens from time to time. The nausea has not been accompanied by vomiting. Her screening laboratory results identify a grade 1 hemoglobin level; all other test results are normal. (2) Assuming this woman is enrolled in the study, what pre-existing conditions would need to be entered into the Medical History e. CRF? 8

This woman is found to be eligible for the study and is enrolled on 10 May 2018. She is randomized to Arm 2. At her Antepartum Week 4 and Week 8 Visits, this participant is found to be well, with no abnormal clinical or laboratory findings. She reports no new symptoms at these visits; she has used her inhaler a few times between visits, but no more often than she did prior to her study participation. She has not had any nausea since enrollment and has taken her study drugs with no reported issues or problems. (3) What updates of this participant’s baseline history would need to be source documented? What new data would need to be entered into e. CRFs? 9

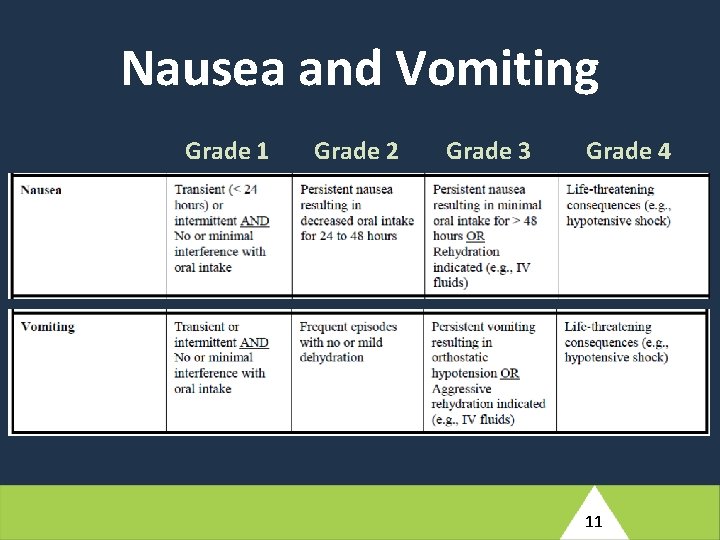
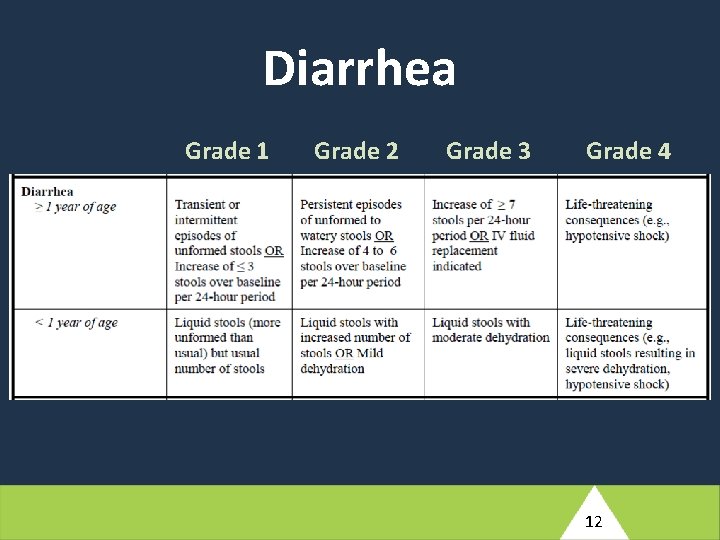
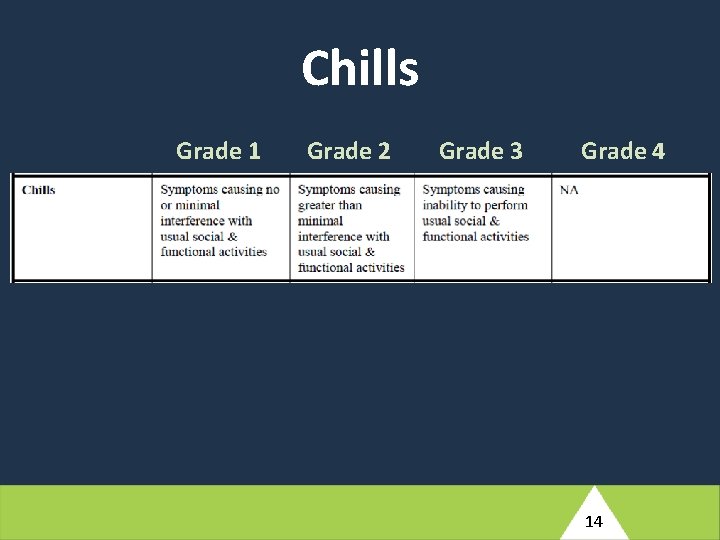
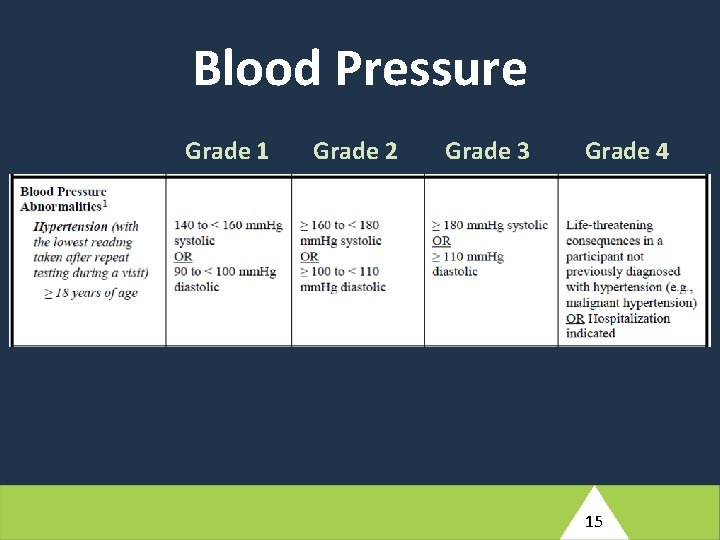
At Antepartum Week 12, this participant reports a history of nausea, vomiting, and diarrhea which started two days ago. She has not taken her temperature but reports periods of feeling hot and having intermittent chills. Her husband has had similar symptoms for the past three days. She has not been able to perform her usual household tasks or eat solid food, but has been able to tolerate small amounts of tea. The study clinician notes that the participant appears unwell and has an axillary measured fever of 39. 2°C. Her blood pressure is 130/92. (4) Based on the brief description provided here, what would your differential diagnosis include for this participant’s reported symptoms? 10

Nausea and Vomiting Grade 1 Grade 2 Grade 3 Grade 4 11

Diarrhea Grade 1 Grade 2 Grade 3 Grade 4 12

Fever 13

Chills Grade 1 Grade 2 Grade 3 Grade 4 14

Blood Pressure Grade 1 Grade 2 Grade 3 Grade 4 15

At Antepartum Week 12, this participant reports a history of nausea, vomiting, and diarrhea which started two days ago. She has not taken her temperature but reports periods of feeling hot and having intermittent chills. Her husband has had similar symptoms for the past three days. She has not been able to perform her usual household tasks or eat solid food, but has been able to tolerate small amounts of tea. The study clinician notes that the participant appears unwell and has an axillary measured fever of 39. 2°C. Her blood pressure is 130/92. (5) How would you manage this participant clinically? What additional evaluations would you perform at the Antepartum Week 12 visit? Would your management involve any changes of ARVs? What would your follow-up plan be? 16

17

At Antepartum Week 12, this participant reports a history of nausea, vomiting, and diarrhea which started two days ago. She has not taken her temperature but reports periods of feeling hot and having intermittent chills. Her husband has had similar symptoms for the past three days. She has not been able to perform her usual household tasks or eat solid food, but has been able to tolerate small amounts of tea. The study clinician notes that the participant appears unwell and has an axillary measured fever of 39. 2°C. Her blood pressure is 130/92. (6) What conditions would need to be entered into the Adverse Event Log e. CRF (ADE 10002)? 18

19

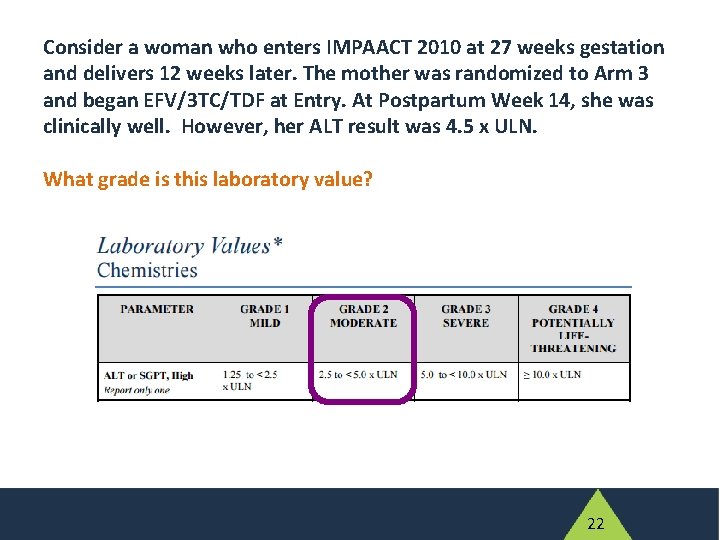
Scenario 2 • Consider a woman who enters IMPAACT 2010 at 27 weeks gestation and delivers 12 weeks later. • The mother was randomized to Arm 3 and began EFV/FTC/TDF at Entry. • At Postpartum Week 14, she was clinically well. However, her ALT result was 4. 5 x ULN. 20

Consider a woman who enters IMPAACT 2010 at 27 weeks gestation and delivers 12 weeks later. The mother was randomized to Arm 3 and began EFV/3 TC/TDF at Entry. At Postpartum Week 14, she was clinically well. However, her ALT result was 4. 5 x ULN. What grade is this laboratory value? A. B. C. D. Grade 1 Grade 2 Grade 3 Grade 4 21

Consider a woman who enters IMPAACT 2010 at 27 weeks gestation and delivers 12 weeks later. The mother was randomized to Arm 3 and began EFV/3 TC/TDF at Entry. At Postpartum Week 14, she was clinically well. However, her ALT result was 4. 5 x ULN. What grade is this laboratory value? 22

Consider a woman who enters IMPAACT 2010 at 27 weeks gestation and delivers 12 weeks later. The mother was randomized to Arm 3 and began EFV/3 TC/TDF at Entry. At Postpartum Week 14, she was clinically well. However, her ALT result was 4. 5 x ULN. What management action would you take based on this result? A. Repeat ALT/AST within 3 -4 business days B. Check for signs of clinical hepatitis C. Check total and direct bilirubin D. All of the above 23

Consider a woman who enters IMPAACT 2010 at 27 weeks gestation and delivers 12 weeks later. The mother was randomized to Arm 3 and began EFV/3 TC/TDF at Entry. At Postpartum Week 14, she was clinically well. However, her ALT result was 4. 5 x ULN. At the follow-up visit, she confirms she had no symptoms and her repeat test confirms the initial Grade 2 ALT result. Should you inform the CMC? A. Yes B. No 24

Consider a woman who enters IMPAACT 2010 at 27 weeks gestation and delivers 12 weeks later. The mother was randomized to Arm 3 and began EFV/3 TC/TDF at Entry. At Postpartum Week 14, she was clinically well. However, her ALT result was 4. 5 x ULN. At the follow-up visit, she confirms she had no symptoms and her repeat test confirms the initial Grade 2 ALT result. In addition, her total bilirubin is 1. 8 x ULN. What management action would you take based on this result? 25

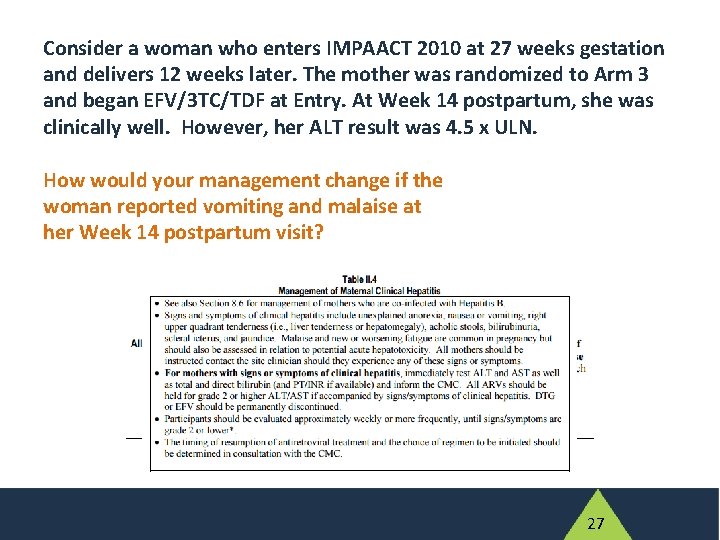
Consider a woman who enters IMPAACT 2010 at 27 weeks gestation and delivers 12 weeks later. The mother was randomized to Arm 3 and began EFV/3 TC/TDF at Entry. At Week 14 postpartum, she was clinically well. However, her ALT result was 4. 5 x ULN. How would your management change if the woman reported vomiting and malaise at her Week 14 postpartum visit? 26

Consider a woman who enters IMPAACT 2010 at 27 weeks gestation and delivers 12 weeks later. The mother was randomized to Arm 3 and began EFV/3 TC/TDF at Entry. At Week 14 postpartum, she was clinically well. However, her ALT result was 4. 5 x ULN. How would your management change if the woman reported vomiting and malaise at her Week 14 postpartum visit? 27

28