Clinical Assessment Reporting and Tracking Program and Research

























- Slides: 27

Clinical Assessment, Reporting and Tracking Program and Research in the Veterans Affairs Healthcare System Javier A. Valle MD MSCS Asst. Director, VA Clinical Assessment, Reporting and Tracking Program Rocky Mountain Regional VA Medical Center Asst. Professor of Medicine University of Colorado School of Medicine

Javier Valle, MD MSCS No relevant disclosures

VA Clinical Assessment, Reporting and Tracking Program Overview • CART Program – Mission, Structure • Reporting: Data, Capabilities – Influencing clinical care for Veterans • Research Opportunities with CART


VA Clinical Assessment, Reporting and Tracking Program The Quality Improvement Imperative The cardiovascular community recognizes the critical importance of structured reporting and calls for its uniform adoption …. . . structured reporting be considered one component of the overall quality improvement imperative for cardiovascular care. AHA/ACC/SCAI 2014 Health policy statement on structured reporting for the cardiac catheterization laboratory. 2014. Journal of the American College of Cardiology , 63: 2591,

VA Clinical Assessment, Reporting and Tracking Program The Quality Improvement Imperative The cardiovascular community recognizes the critical importance of structured reporting and calls for its uniform adoption …. . . structured reporting be considered one component of the overall quality improvement imperative for cardiovascular care. AHA/ACC/SCAI 2014 Health policy statement on structured reporting for the cardiac catheterization laboratory. 2014. Journal of the American College of Cardiology , 63: 2591,

VA Clinical Assessment, Reporting and Tracking Program Mission To monitor and enhance the quality and safety of invasive cardiac procedures for Veterans through clinical analytics and information technology.

VA Clinical Assessment, Reporting and Tracking Program Structure Quality Analytics Outcomes Safety FDA Reporting MAE Information Technology Internal External

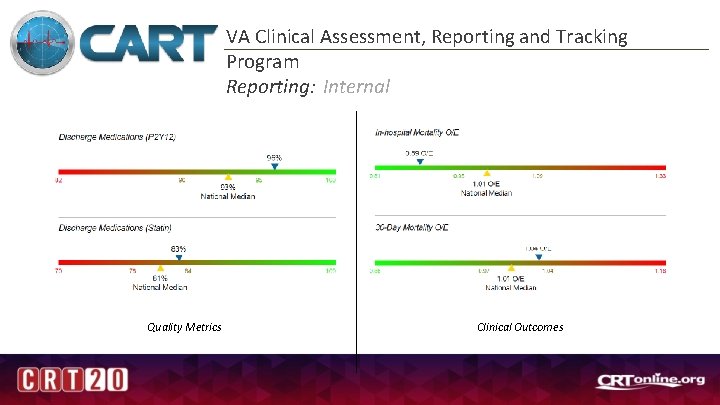
VA Clinical Assessment, Reporting and Tracking Program Reporting: Internal Quality Metrics Clinical Outcomes

VA Clinical Assessment, Reporting and Tracking Program Reporting: External

VA Clinical Assessment, Reporting and Tracking Program Application Suite, Data

VA Clinical Assessment, Reporting and Tracking Program Influencing VA Care Quality Analytics Outcomes Safety FDA Reporting MAE Information Technology Internal External

VA Clinical Assessment, Reporting and Tracking Program Quality: Outcomes

VA Clinical Assessment, Reporting and Tracking Program Quality: Outcomes

VA Clinical Assessment, Reporting and Tracking Program Quality: Outcomes

VA Clinical Assessment, Reporting and Tracking Program Quality: Analytics

VA Clinical Assessment, Reporting and Tracking Program Quality: Analytics

VA Clinical Assessment, Reporting and Tracking Program Quality: Analytics

VA Clinical Assessment, Reporting and Tracking Program Quality: Analytics

VA Clinical Assessment, Reporting and Tracking Program Quality: Analytics

VA Clinical Assessment, Reporting and Tracking Program Pathways for Research: Proposals

VA Clinical Assessment, Reporting and Tracking Program Pathways for Research: Funding Unfunded Research Funded Research Investigators apply for analytic support from CART’s analytic center Investigators provide funds to support analytic process

VA Clinical Assessment, Reporting and Tracking Program Funded Research Submission: Submission of research proposal Process: Preliminary analytic budget provided to principal investigator Grant liaison will work with investigator to finalize subcontracting Funded Research Funding: Dependent upon external funding agency Contracting: Denver Research Institute (NIH / Foundation / Industry Funding) Rocky Mountain Regional VA Medical Center Research (VA Funding) Execution: Analytic work will be performed by the contracting analytic center Aggregate results will be provided to principal investigator

VA Clinical Assessment, Reporting and Tracking Program Submission: Available to anyone with at least a 5/8 th appointment at any VA Facility Unfunded Research Submission of research proposal Primary authors may have only one unfunded project in progress at any time Process: Proposals undergo internal feasibility & duplication review Feasible proposals without duplication proceed to scoring by independent review committee Unfunded Research Funding: Independent committee meets twice each year Proposals are scored based on priority, funding determined by analytic needs, resources, and priorities at time of cycle Contracting: CART Program liaison will contact funded investigators Execution: Analytic work will be performed by the contracting analytic center Aggregate results will be provided to principal investigator

VA Clinical Assessment, Reporting and Tracking Program Pathways for Research Funded Research: -Grant Budgeting -Contracting process Proposal Submission Feasibility/ Duplication Review VA IRB Submission Unfunded Research: -R&P Committee review Analytic Plan Proposed Analysis Manuscript Review+ Submission

VA Clinical Assessment, Reporting and Tracking Program Summary CART is a valuable resource for scientific investigation Opportunities for investigators to perform high value research and influence Veteran care

Thank you! Questions? Javier Valle javier. valle 3@va. gov