Clindamycin vs Bactrim in the Treatment of Soft

Clindamycin vs. Bactrim in the Treatment of Soft Skin Tissue Infections Jenny Chan R 1 Journal Club 3/25/15

Overview Case Presentation Background Journal Article Critical Appraisal Discussion Limitations Conclusion

Case Presentation 52 year old female with HTN, HLD, DM type 2, and ESRD on HD who presented with a skin lesion on the left arm S: 5 days prior began as a small blister ruptured and began to increase in size and level of pain O: Afebrile & normal vitals. 4 x 3. 5 cm superficial abrasion on the left arm with surrounding erythema, tenderness and warmth but no drainage or fluctuance What is the best way to treat her skin infection?

Background
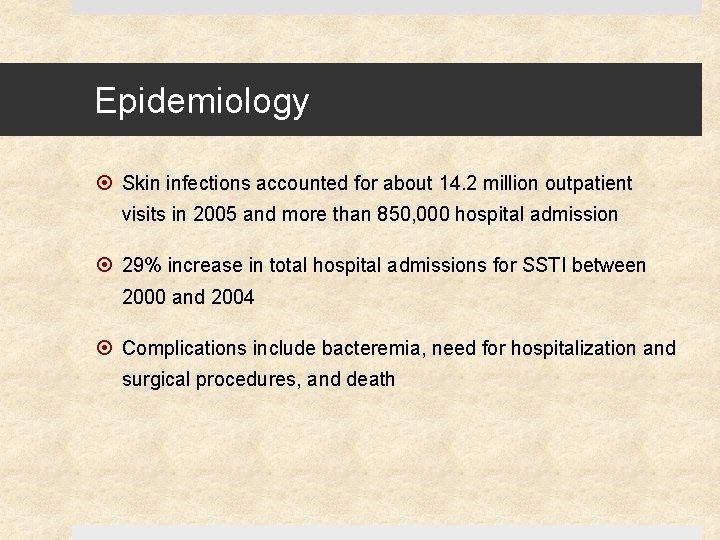
Epidemiology Skin infections accounted for about 14. 2 million outpatient visits in 2005 and more than 850, 000 hospital admission 29% increase in total hospital admissions for SSTI between 2000 and 2004 Complications include bacteremia, need for hospitalization and surgical procedures, and death
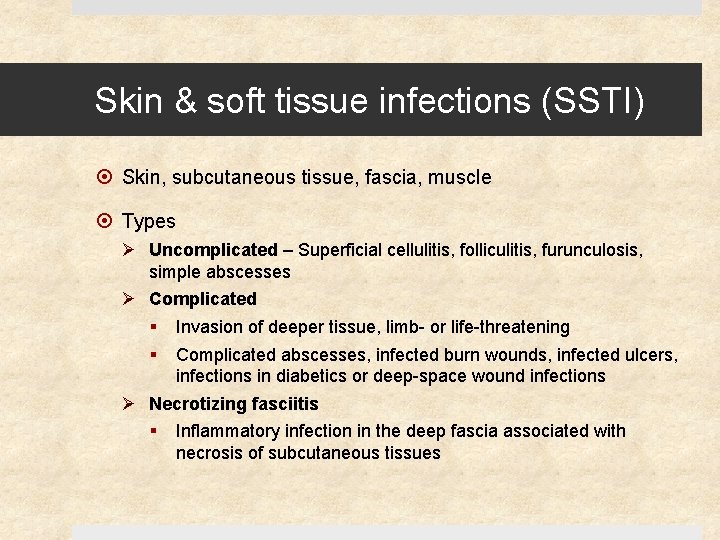
Skin & soft tissue infections (SSTI) Skin, subcutaneous tissue, fascia, muscle Types Ø Uncomplicated – Superficial cellulitis, folliculitis, furunculosis, simple abscesses Ø Complicated § Invasion of deeper tissue, limb- or life-threatening § Complicated abscesses, infected burn wounds, infected ulcers, infections in diabetics or deep-space wound infections Ø Necrotizing fasciitis § Inflammatory infection in the deep fascia associated with necrosis of subcutaneous tissues
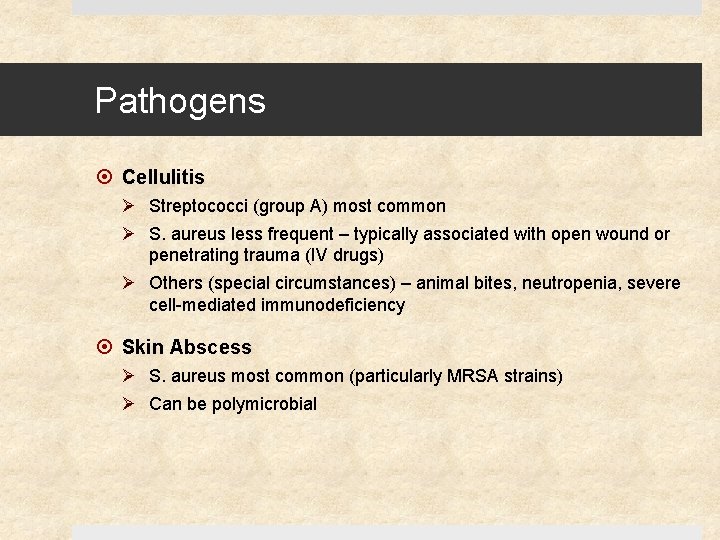
Pathogens Cellulitis Ø Streptococci (group A) most common Ø S. aureus less frequent – typically associated with open wound or penetrating trauma (IV drugs) Ø Others (special circumstances) – animal bites, neutropenia, severe cell-mediated immunodeficiency Skin Abscess Ø S. aureus most common (particularly MRSA strains) Ø Can be polymicrobial
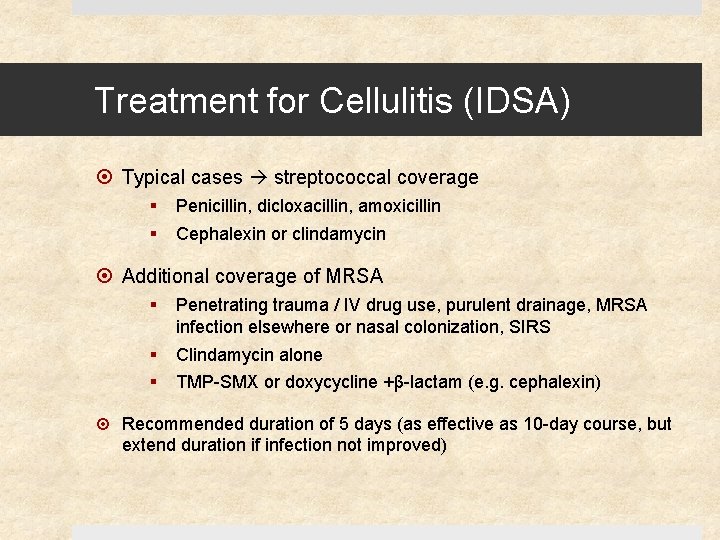
Treatment for Cellulitis (IDSA) Typical cases streptococcal coverage § § Penicillin, dicloxacillin, amoxicillin Cephalexin or clindamycin Additional coverage of MRSA § Penetrating trauma / IV drug use, purulent drainage, MRSA infection elsewhere or nasal colonization, SIRS § § Clindamycin alone TMP-SMX or doxycycline +β-lactam (e. g. cephalexin) Recommended duration of 5 days (as effective as 10 -day course, but extend duration if infection not improved)
Treatment for Abscess (IDSA) I&D with gram stain & culture § I&D alone may be sufficient for abscess < 5 cm Antibiotics § S. aureus / MRSA coverage § Recommended if systemic signs (SIRS), multiple abscesses, rapid progression & associated w/ cellulitis, areas difficult to drain (face, hand, genitalia), comorbidities or immunosuppression, extremes of age, lack of response to I&D § Does not improve cure rates, but may affect time to recurrence of other abscesses
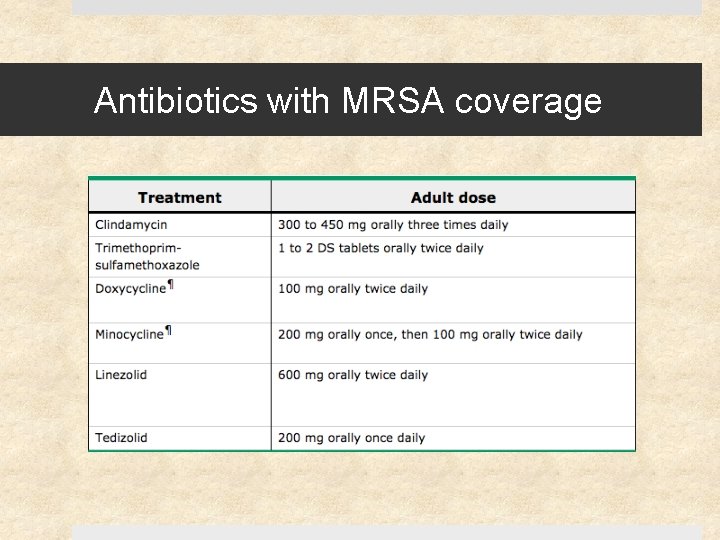
Antibiotics with MRSA coverage

Antibiotics Clindamycin Ø Good activity against MRSA and strep Ø Good tissue penetration (particularly bone & abscesses) Ø Inhibits toxin production Ø Bacteriostatic Ø Resistance rates § Some avoid use for empiric tx if rates > 10 -15% § May have resistance in erythromycin-resistant strains Ø Poor gram neg activity but covers anaerobes TMP-SMX Ø Not advised for empiric tx of group A strep infections Ø Bactericidal Ø 95 -100% of CA-MRSA strains are susceptible in vitro Ø Caution in elderly, especially w/ RAS inhibitors or chronic renal insufficiency (risk of hyperkalemia) Ø Has gram neg activity but not anaerobic coverage
Antibiotics Tetracyclines Linezolid Ø Not advised for empiric tx of group A strep infections Ø Limited by cost and toxicity Ø Bacterostatic Ø Used as alternative, but limited clinical experience Ø Not recommended for < 8 yo

Funded by the National Institutes of Health The National Institute of Allergy and Infectious Disease The National Center for Advancing Translational Sciences

Objective To compare the efficacy between clindamycin and TMP-SMX in the treatment of uncomplicated skin infections

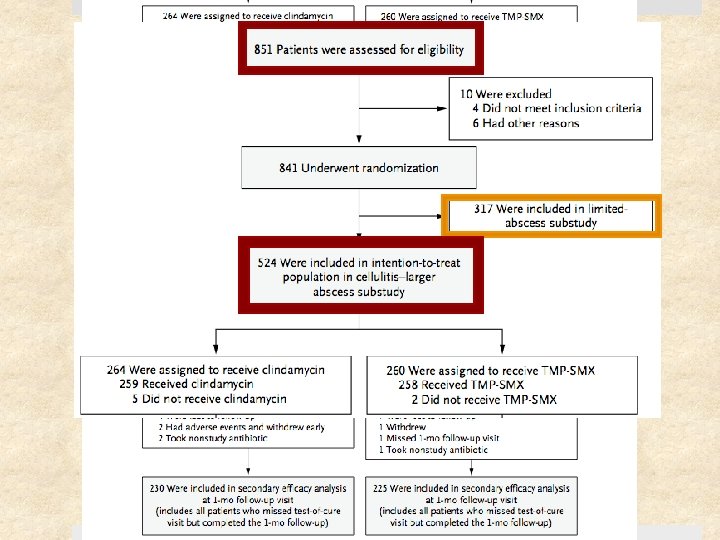
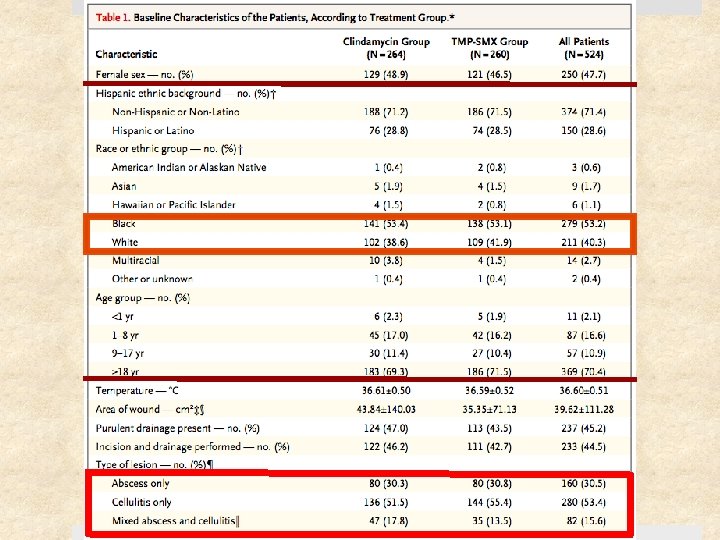
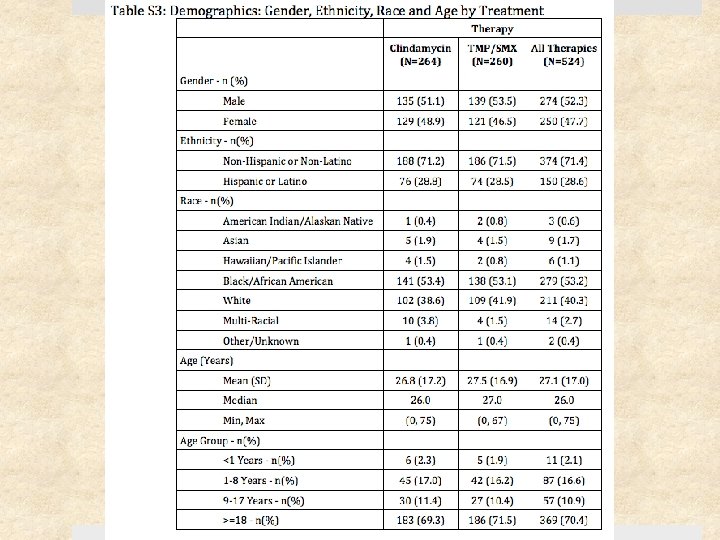
Study Design Prospective, randomized, double-blinded clinical trial Setting Ø May 2009 – August 2011 Ø Urgent care clinics, ED, and affiliated clinics Ø 4 institutions: University of Chicago, SF General Hospital, Harbor. UCLA, Vanderbilt University Patients: 524 patients (children & adults) with cellulitis and/or skin abscess Follow-up period: 1 month after treatment completion
Selection Criteria Inclusion Criteria Exclusion Criteria > 2 signs or symptoms for at least 24 hr: Ø Erythema Ø Swelling or induration Ø Warmth Ø Purulent drainage Ø Tenderness or pain Superficial infection (impetigo) Requiring specialized management (e. g. genital), surgical or prosthetic device infection Human or animal bite Fever > 38. 5°C or 38°C (<1 yo) Age 6 mo – 85 yrs On immunosuppressants, CA requiring tx within past year, living in long-term care facility Able to take oral abx DM, CKD, or BMI > 40 Received abx w/ antistaph activity in the past 14 days
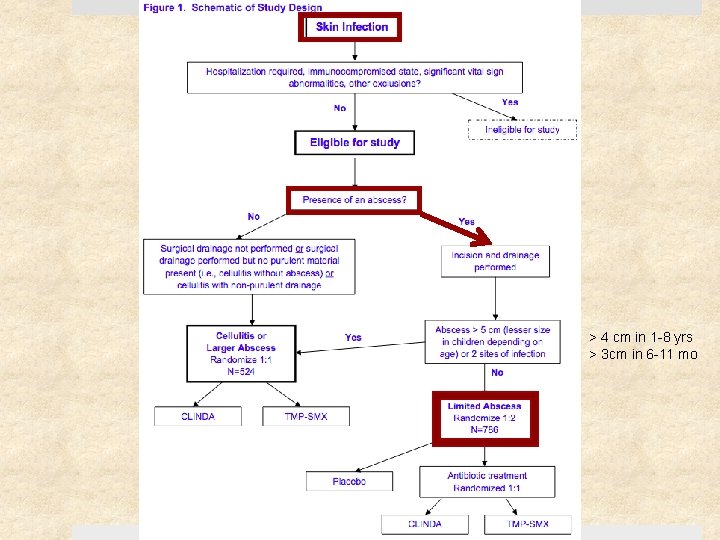
> 4 cm in 1 -8 yrs > 3 cm in 6 -11 mo
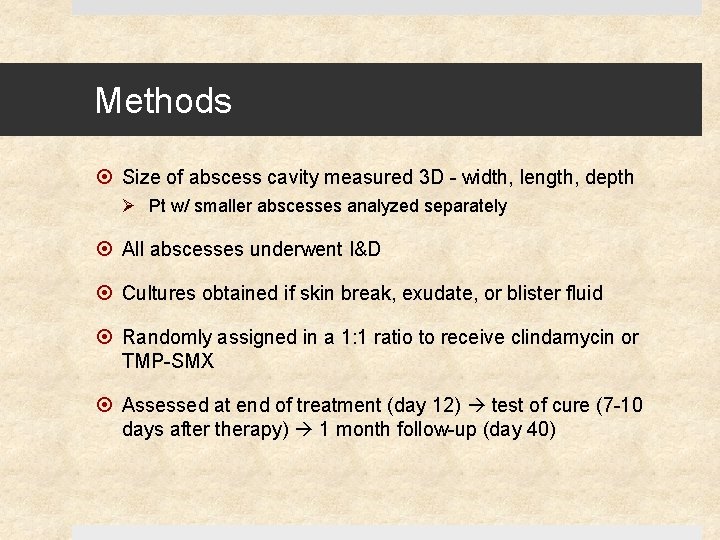
Methods Size of abscess cavity measured 3 D - width, length, depth Ø Pt w/ smaller abscesses analyzed separately All abscesses underwent I&D Cultures obtained if skin break, exudate, or blister fluid Randomly assigned in a 1: 1 ratio to receive clindamycin or TMP-SMX Assessed at end of treatment (day 12) test of cure (7 -10 days after therapy) 1 month follow-up (day 40)
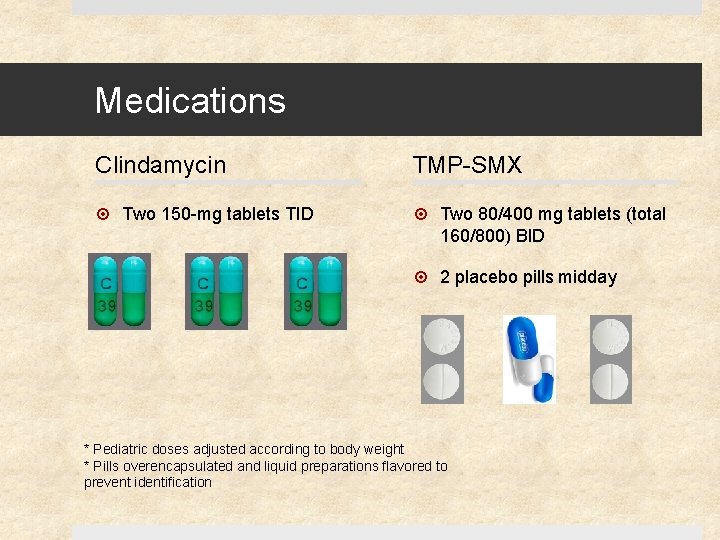
Medications Clindamycin TMP-SMX Two 150 -mg tablets TID Two 80/400 mg tablets (total 160/800) BID 2 placebo pills midday * Pediatric doses adjusted according to body weight * Pills overencapsulated and liquid preparations flavored to prevent identification
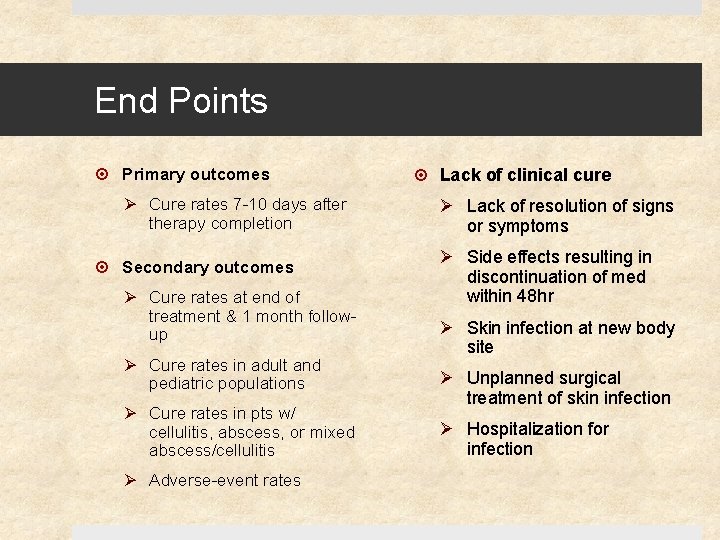
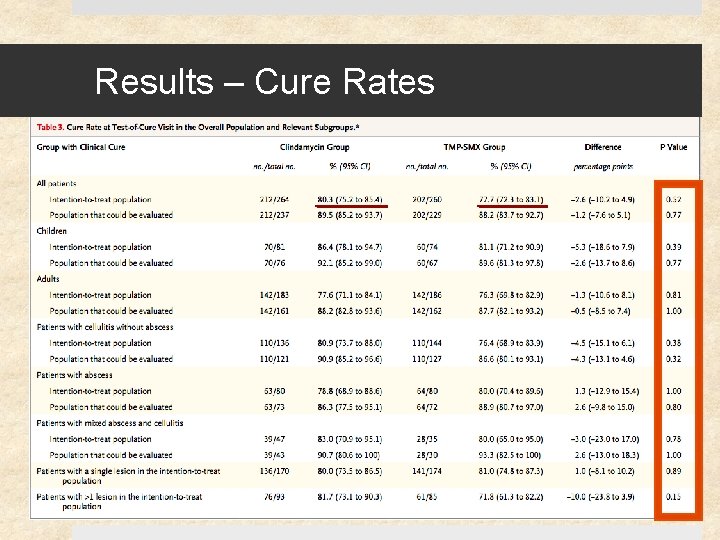
End Points Primary outcomes Ø Cure rates 7 -10 days after therapy completion Secondary outcomes Ø Cure rates at end of treatment & 1 month followup Ø Cure rates in adult and pediatric populations Ø Cure rates in pts w/ cellulitis, abscess, or mixed abscess/cellulitis Ø Adverse-event rates Lack of clinical cure Ø Lack of resolution of signs or symptoms Ø Side effects resulting in discontinuation of med within 48 hr Ø Skin infection at new body site Ø Unplanned surgical treatment of skin infection Ø Hospitalization for infection
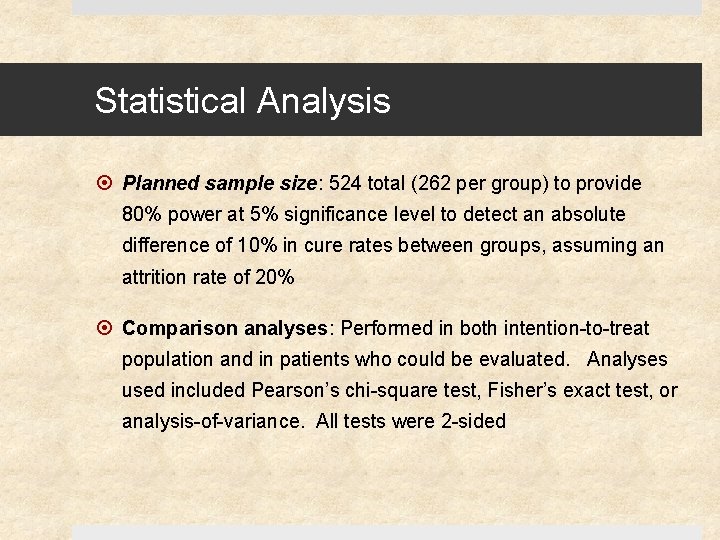
Statistical Analysis Planned sample size: 524 total (262 per group) to provide 80% power at 5% significance level to detect an absolute difference of 10% in cure rates between groups, assuming an attrition rate of 20% Comparison analyses: Performed in both intention-to-treat population and in patients who could be evaluated. Analyses used included Pearson’s chi-square test, Fisher’s exact test, or analysis-of-variance. All tests were 2 -sided

Results
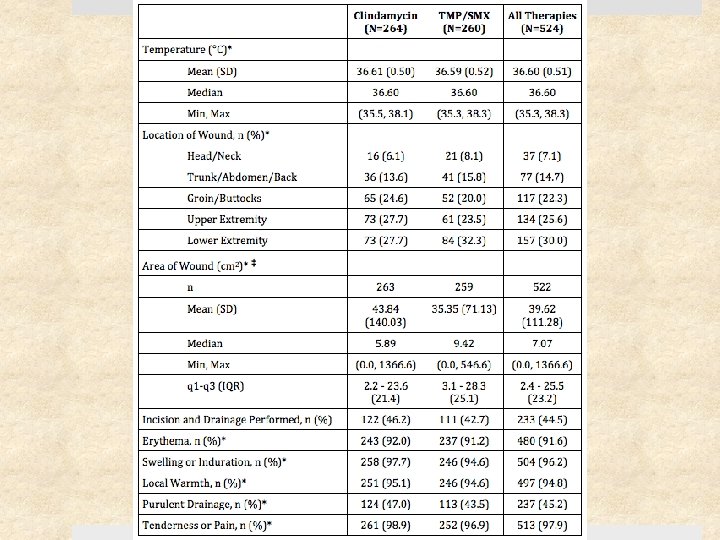
Results – Cure Rates
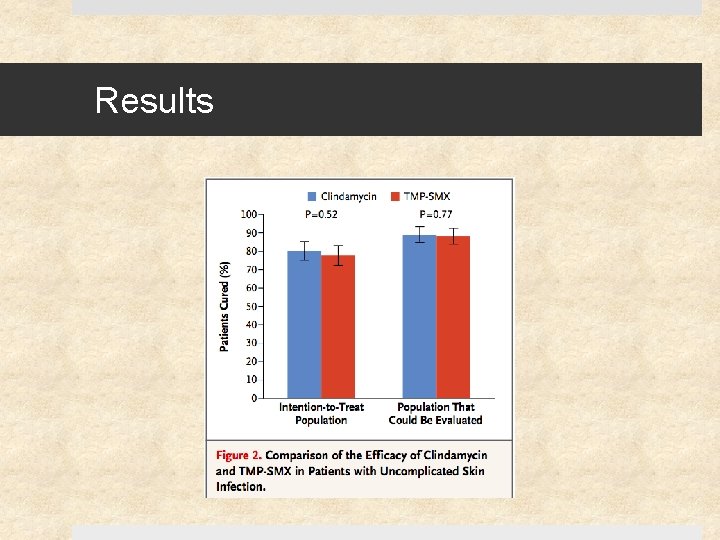
Results
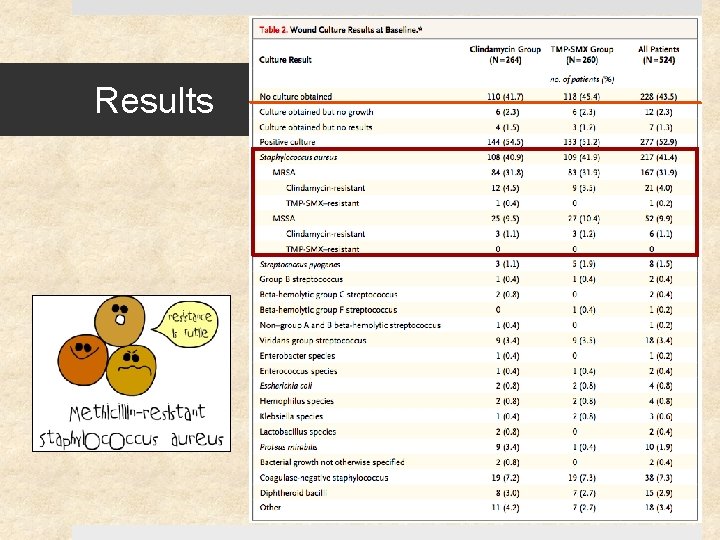
Results
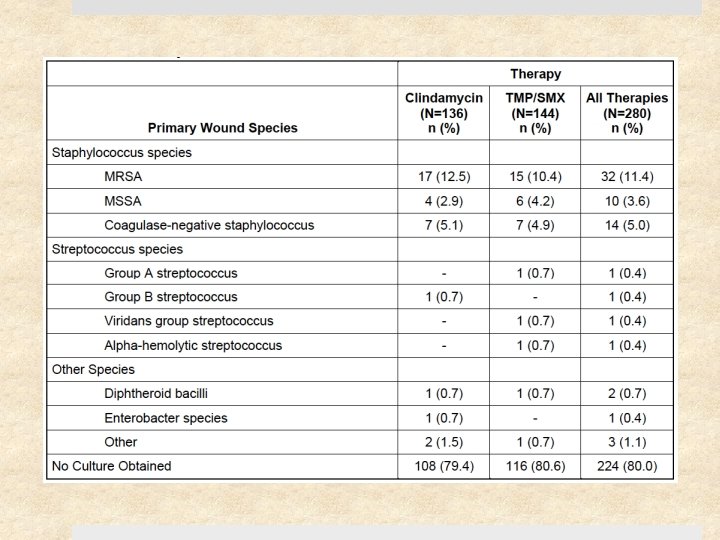
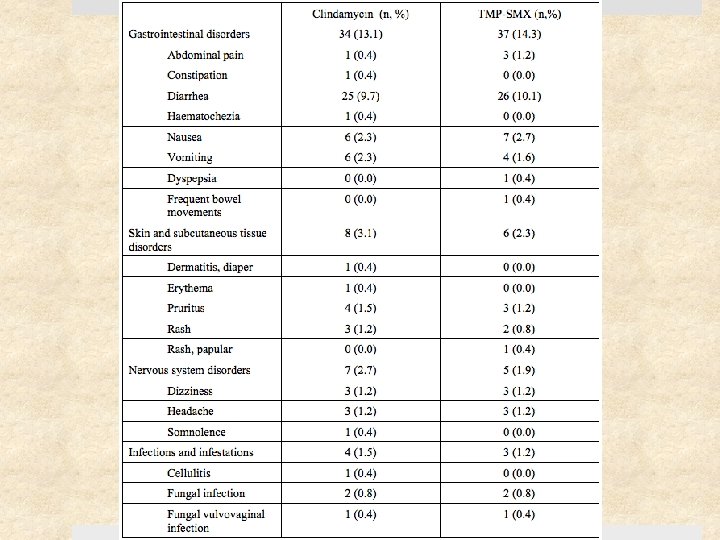
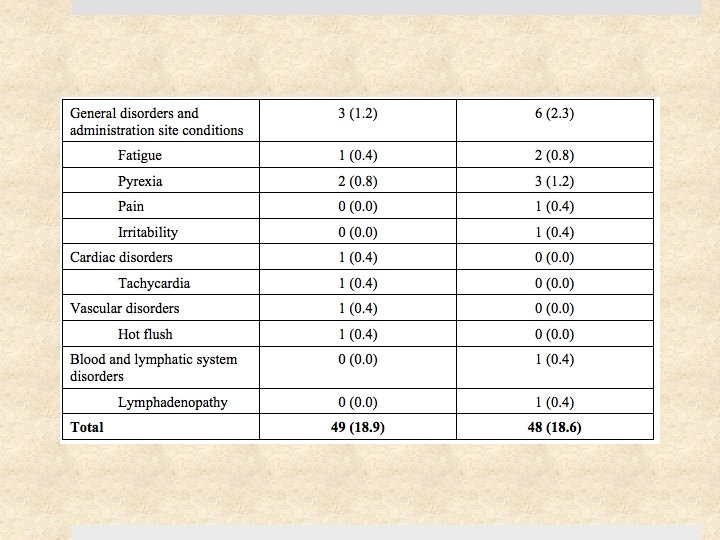
Results 41% (217) of study patients had S. aureus Ø 12% (27) of these were clindamycin-resistant Ø 0. 5% (1) of these were TMP-SMX resistant 11 of 15 clindamycin-treated patients with clindamycin-resistant S. aureus were cured (73% [47 – 99. 7%]) Cure rates at 1 month were similar for clindamycin and TMPSMX: difference of -5. 7% [-13. 3 to 1. 9], p=0. 15 Rate of adverse events similar (18%) – diarrhea (10%) was most common (but no C. difficile cases), followed by nausea, vomiting, pruritis, and rash. No serious events.

Critical Appraisal Randomized: Yes Double Blinded: Yes Multicenter: Yes Similar Baseline Characteristics: Yes Groups treated equally: Yes All patients accounted for? : Yes

Discussion No significant difference between clindamycin and TMP-SMX in efficacy for the treatment of uncomplicated skin infections, including cellulitis and abscesses No significant difference in rate of adverse events between the 2 groups
Limitations Excluded population with comorbidities (DM, CKD, etc) Excluded smaller abscesses Effect of I&D alone on cure rate of patients with larger abscess unknown Sample size not large enough to evaluate subgroup of cellulitis* or cure rate for clindamycin-resistant S. aureus Short follow-up (1 month may be inadequate to assess recurrence of S. aureus) Other antibiotics not tested
Choosing Antibiotics Co-morbidities (e. g. renal function) or concurrent meds Clinical history and presentation Antibiotic susceptibility profiles Weight (dosing) Cost Compliance
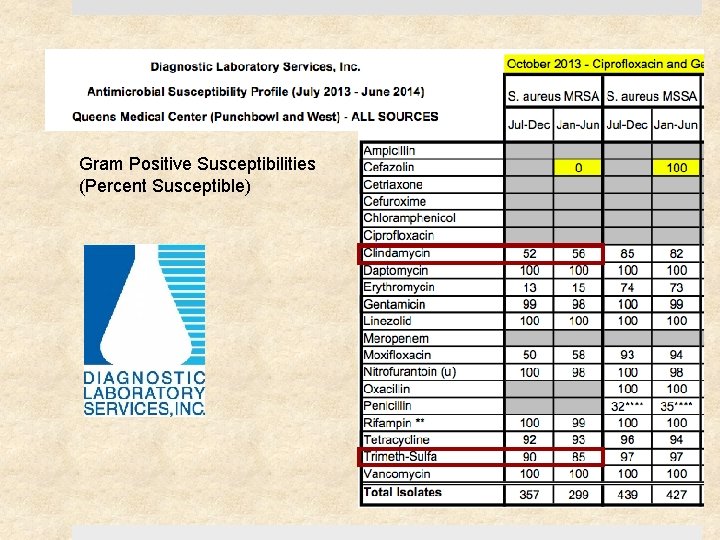
Gram Positive Susceptibilities (Percent Susceptible)

Will these results help my patient? Can the results be applied to my patient? Ø My patient does not fit the inclusion criteria (she has DM and ESRD) or demographics (ethnicity) Were all clinically important outcomes considered? Yes Are the likely benefits worth the potential harms & cost? Ø Yes. Adverse effects mostly GI related, but unable to assess possible harms in patients with more co-morbidities
References Miller L, et al. Clindamycin versus Trimethoprim-Sulfamethoxazole for Uncomplicated Skin Infections. March 2015. N Engl J Med 372: 12 Stevens D, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. June 2014 Liu C, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus Aureus Infections in Adults and Children. Jan 2011. Lowy F. Treatment of skin and soft tissue infections due to MRSA in adults. [Up. To. Date] Dec 2014.

Thank You!
- Slides: 41