Click to edit Master title style Edit Master












- Slides: 52

Click to edit Master title style • Edit Master text styles • Second level Lipid-L 6 • Third level • Fourth level • Fifth level Lipid Metabolism 1

Click to edit Master title style Cholesterol and Steroids Metabolism • Edit Master text styles • Second level • Third level • Fourth level • Fifth level Prof. Dr. Randa Ali-Labib Prof. Dr. Hanan Shehata Medical Biochemistry and Molecular Biology Department 2

Click to edit Master title style Intended Learning Outcomes Edit Master textthis styleslecture , the student should be By • the end of level able • Second to • describe the: Third level • Fourth level 1. Different types of Steroid lipids. • Fifth level 2. Role of some steroids. 3. Structure of some steroids. 4. Synthesis and degradation of steroids. 5. Synthesis of bile. 6. Some abnormalities in Steroid lipid metabolism ©

Click to edit Master title style Cholesterol and • Edit Master text styles. Steroid Metabolism topics: • Second level I. Structure of Sterols and Cholesterol • Third level II. Liver cholesterol pool Influx and Efflux • Fourth level III. Synthesis of Cholesterol. • Fifth level A. Synthesis of HMG-Co. A B. Synthesis of mevalonate C. Synthesis of cholesterol IV. Regulation of cholesterol synthesis A. Sterol-dependent regulation of gene expression B. Sterol-accelerated enzyme degradation C. Sterol-independent phosphorylation/dephosphorylation D. Hormonal regulation E. Inhibition by drugs

Cholesterol and Steroid Click to edit Master title style. Metabolism topic Cont. , V. Master Degradation • Edit text styles of Cholesterol VI. Bile Acids and Bile Salts • Second level • Third. A. level Structure of the bile acids • Fourth level B. • Synthesis of bile acids Fifth level C. Synthesis of bile salts D. Action of intestinal flora on bile salts E. Enterohepatic circulation F. Bile salt deficiency: cholelithiasis

Click to edit Master title style I-Structure of Sterols And Cholesterol • Edit Master text styles • Second level • Third level • Fourth level • Fifth level

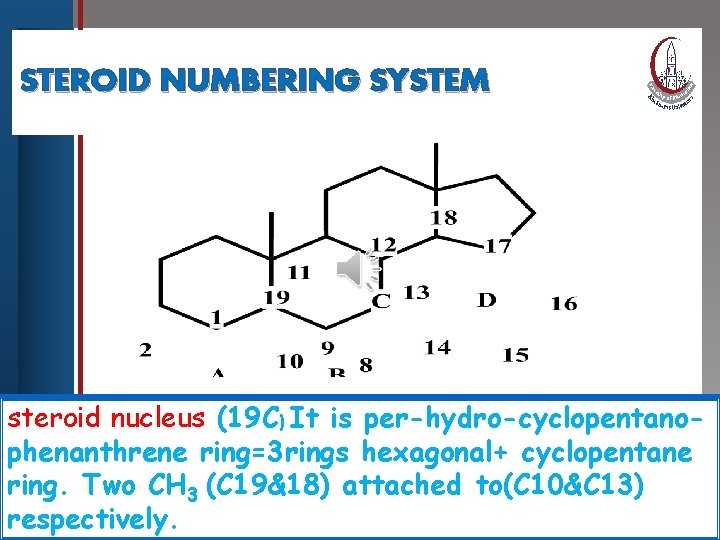
STEROID NUMBERING Click to edit Master title. SYSTEM style • Edit Master text styles • Second level • Third level • Fourth level • Fifth level steroid nucleus (19 C) It is per-hydro-cyclopentanophenanthrene ring=3 rings hexagonal+ cyclopentane ring. Two CH 3 (C 19&18) attached to(C 10&C 13) respectively.

Click to edit Master title style Important Steroids are: • Edit Master text styles • Second level 1 -Cholesterol (27 C) (Later discussed in Details) • Third level • Fourth level • Fifth level 2 -Steroid hormones: Synthesized from cholesterol in specific tissues, differs in structure and action. They are 2 classes: Sex hormones & adrenal hormones (Glucocorticoides & Mineralocorticoides)

3 -Bile acids (24 C): Click to editfrom Master title styledirectly by -Synthesized cholesterol hepatocytes and excreted in bile. - Having a 5 C side chain ending with COOH • Edit Master text styles Second level conjugated to either glycine or which • is often • Third level taurine (3; 1) tolevelform glycocholic and • Fourth • Fifth level Taurocholic acid. -They haven’t got double bond at C 5 4 -Plant Sterols (28 C): As cholesterol in having OH at. C 3 -Are poorly absorbed by humans. -It is converted to Vit D 2 (ergocalciferol) by the action of UV.

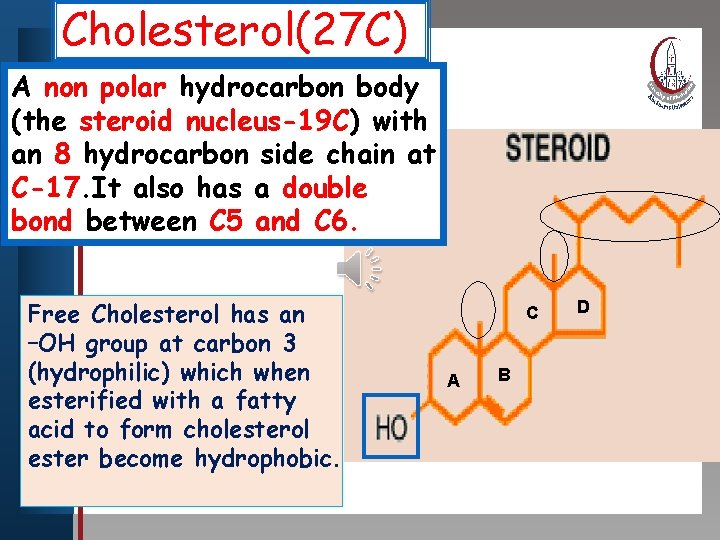
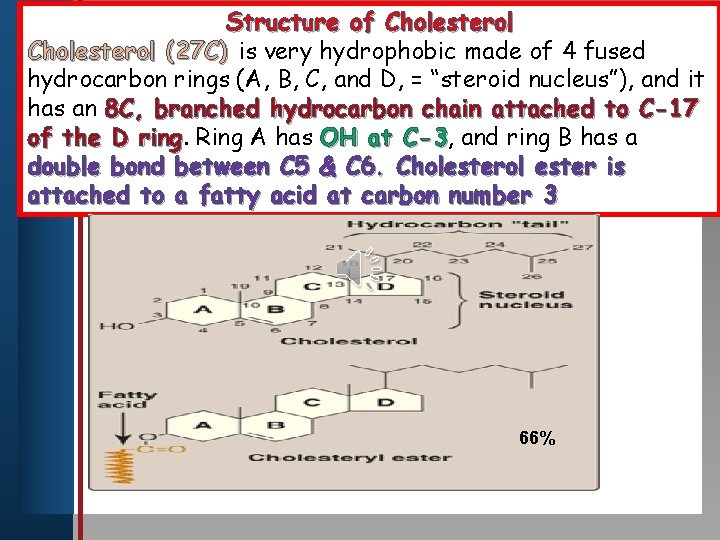
Cholesterol(27 C) Clickpolar to edit Master titlebody style A non hydrocarbon (the steroid nucleus-19 C) with an 8 hydrocarbon • Edit Master text stylesside chain at • Second levelhas a double C-17. It also • Third level bond between C 5 • Fourth level and C 6. • Fifth level Free Cholesterol has an –OH group at carbon 3 (hydrophilic) which when esterified with a fatty acid to form cholesterol ester become hydrophobic. C A B D

Structure of Cholesterol (27 C) is very hydrophobic made of 4 fused hydrocarbon rings. Master (A, B, C, title and style D, = “steroid nucleus”), and it Click to edit has an 8 C, branched hydrocarbon chain attached to C-17 of the D ring Ring A has OH at C-3, C-3 and ring B has a • Edit bond Master between text styles C 5 & C 6. Cholesterol ester is double • Second level attached • to a fatty acid at carbon number 3 Third level • Fourth level • Fifth level 66%

Click to edit Master title style II-Liver cholesterol pool Influx and Efflux • Edit Master text styles • Second level • Third level • Fourth level • Fifth level

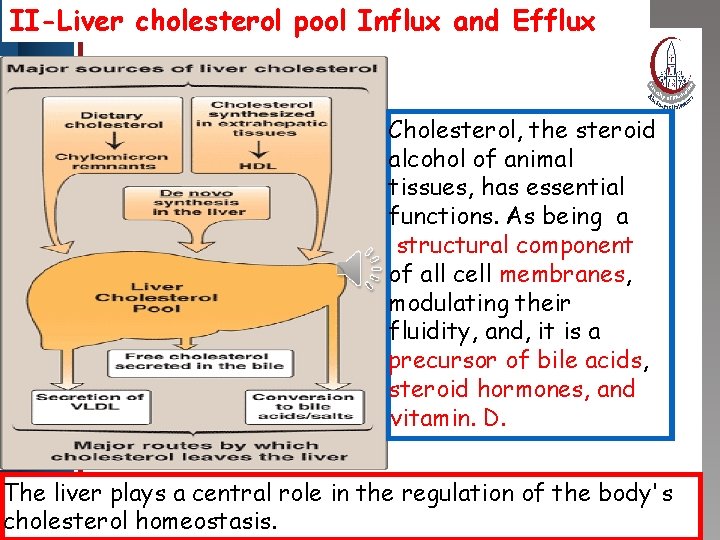
II-Liver cholesterol pool Influx and Efflux Click to edit Master title style • Edit Master text styles • Second level • Third level • Fourth level • Fifth level Cholesterol, the steroid alcohol of animal tissues, has essential functions. As being a structural component of all cell membranes, modulating their fluidity, and, it is a precursor of bile acids, steroid hormones, and vitamin. D. The liver plays a central role in the regulation of the body's cholesterol homeostasis.

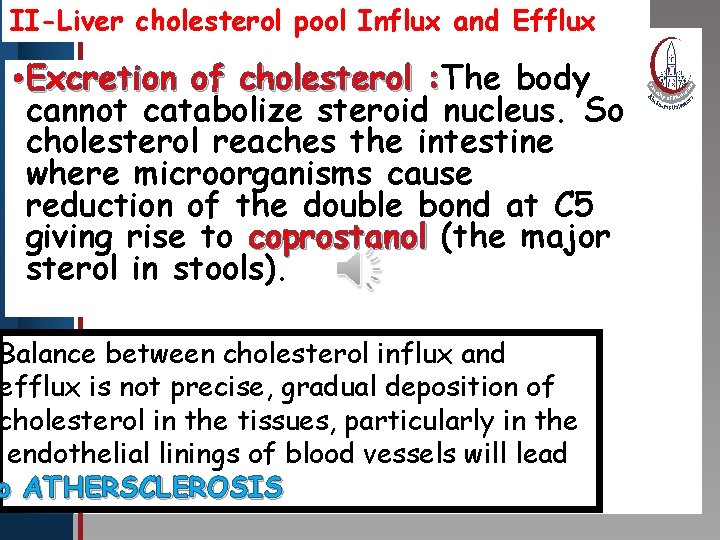
II-Liver cholesterol pool Influx and Efflux • Excretion of. Master cholesterol body : Click to edit title style: The cannot catabolize steroid nucleus. So cholesterol reaches the intestine • Edit Master text styles where microorganisms cause • Second level • Third level reduction of the double bond at C 5 • Fourth level giving rise • to coprostanol (the major Fifth level sterol in stools). Balance between cholesterol influx and efflux is not precise, gradual deposition of cholesterol in the tissues, particularly in the endothelial linings of blood vessels will lead o ATHERSCLEROSIS

Click to edit Master title style • Edit Master text styles • Second level III. Synthesis of Cholesterol A. Synthesis of HMG-Co. A B. Synthesis of mevalonate C. Synthesis of cholesterol • Third level • Fourth level • Fifth level

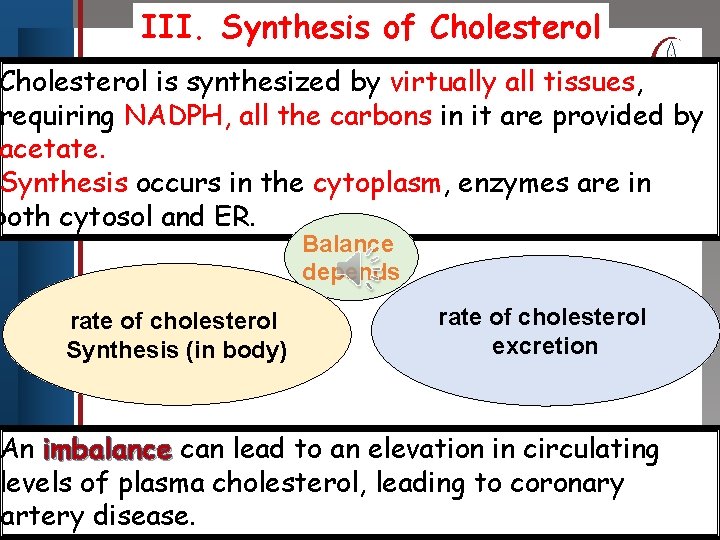
III. Synthesis of Cholesterol Click to is edit Master title Cholesterol synthesized bystyle virtually all tissues, requiring NADPH, all the carbons in it are provided by acetate. • Edit Master text styles • Second level in the cytoplasm, enzymes are in Synthesis occurs • Third level both cytosol and • Fourth. ER. level • Fifth level rate of cholesterol Synthesis (in body) Balance depends rate of cholesterol excretion An imbalance can lead to an elevation in circulating levels of plasma cholesterol, leading to coronary artery disease.

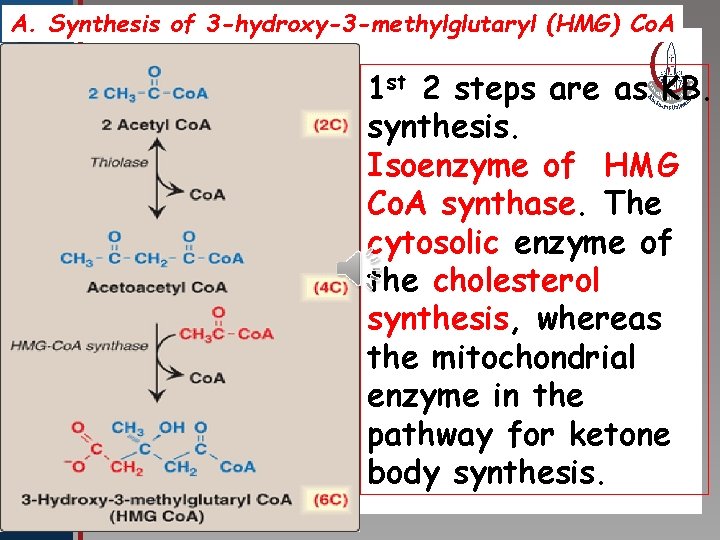
A. Synthesis of 3 -hydroxy-3 -methylglutaryl (HMG) Co. A st 2 steps are as KB. Click to edit Master title 1 style synthesis. • Edit Master text styles Isoenzyme of HMG • Second level • Third level Co. A synthase. The • Fourth level • Fifth level cytosolic enzyme of the cholesterol synthesis, whereas the mitochondrial enzyme in the pathway for ketone body synthesis.

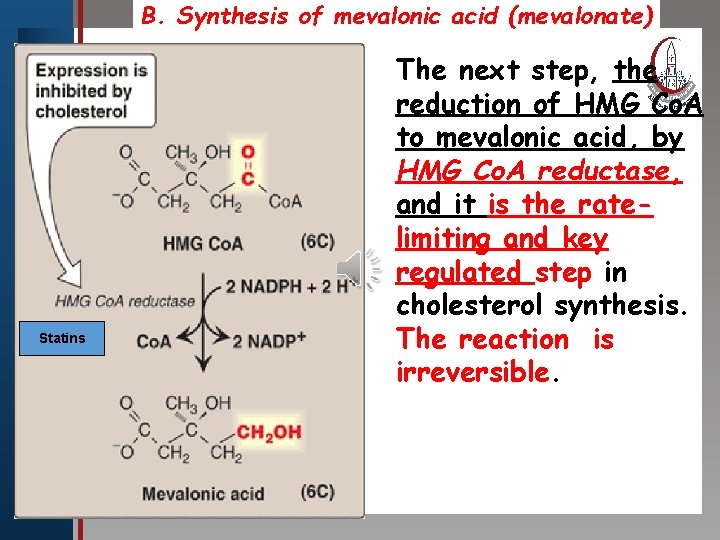
B. Synthesis of mevalonic acid (mevalonate) The next step, the Click to edit Master title style • Edit Master text styles • Second level • Third level • Fourth level • Fifth level Statins reduction of HMG Co. A to mevalonic acid, by HMG Co. A reductase, and it is the ratelimiting and key regulated step in cholesterol synthesis. The reaction is irreversible.

C. Synthesis of cholesterol Click to edit Master title style Then from the last step in which • Second level mevalonic acid (mevalonate), several steps will occurs for cholesterol synthesis as follows: • Edit Master text styles • Third level • Fourth level • Fifth level

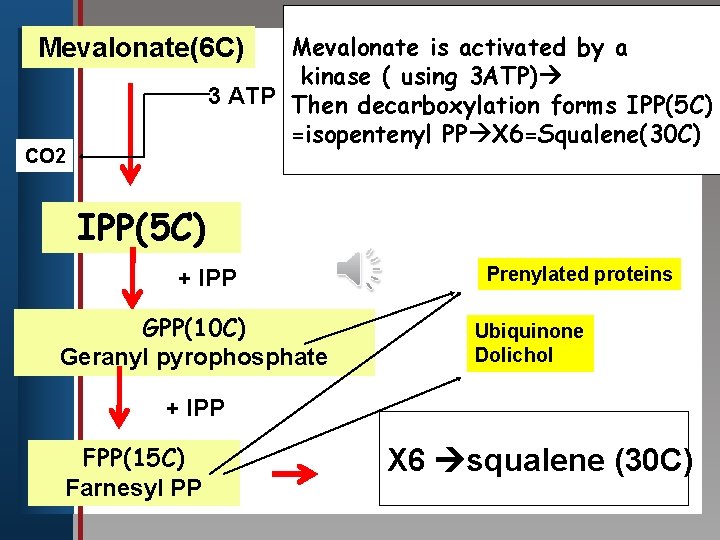
Mevalonate is activated by a kinase ( using 3 ATP) edit 3 Master title style ATP Then decarboxylation forms IPP(5 C) =isopentenyl PP X 6=Squalene(30 C) Mevalonate(6 C) Click to CO 2 • Edit Master text styles • Second level • Third level IPP(5 C) • Fourth level • Fifth level + IPP GPP(10 C) Geranyl pyrophosphate Prenylated proteins Ubiquinone Dolichol + IPP FPP(15 C) Farnesyl PP X 6 squalene (30 C)

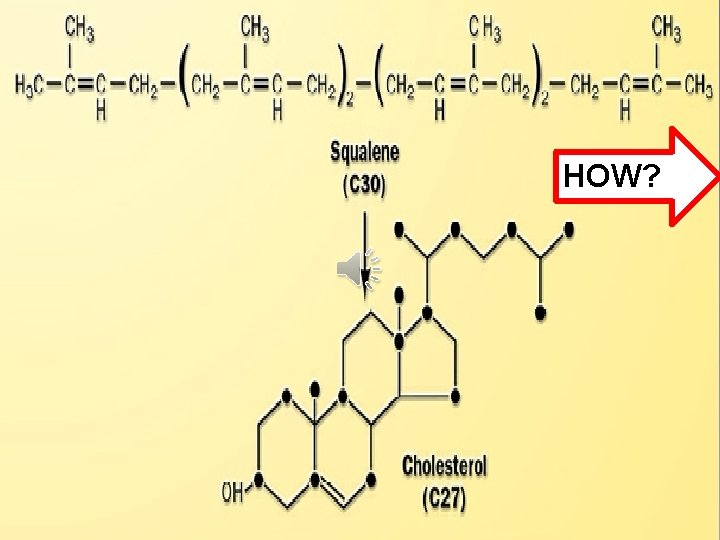
Click to edit Master title style • Edit Master text styles • Second level • Third level • Fourth level • Fifth level HOW?

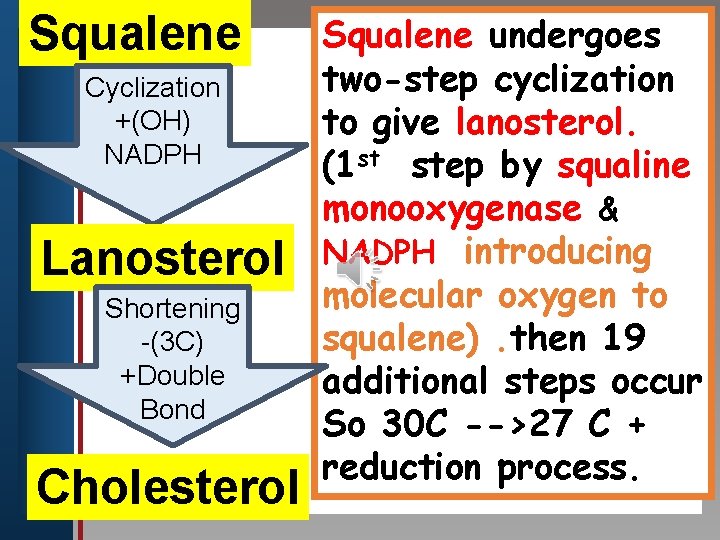
Squalene undergoes two-step cyclization Click to edit Master title style Cyclization +(OH) to give lanosterol. • Edit. NADPH Master text styles st step by squaline (1 • Second level • Third level monooxygenase & Lanosterol NADPH introducing molecular oxygen to Shortening squalene). then 19 -(3 C) +Double additional steps occur Bond So 30 C -->27 C + reduction process. • Fourth level • Fifth level Cholesterol

Summary of Cholesterol Click to edit Master title style synthesis stages • Synthesis of HMGCo. A from acetyl Co. A • Edit Master text styles • Conversion HMGCo. A to mevalonate • Secondof level • Third • Conversion of level mevalonate to activated isoprene unit (C 5) • Fourth level • Condensation of • 6 Fifth activated isoprene units to form squalene level (C 30) • Conversion of squalene to lanosterol • Conversion of lanosterol to cholesterol (C 27) Normal cholesteol synthesis is 1. 0 g/day& consumption is about 0. 3 g/day. The normal body level is (140 -200 mg%). Although cholesterol is of importance , High levels are risky. Regulation is through both diet and (HMG COA reducase)

Click to edit Master title style IV. • Edit Regulation of cholesterol synthesis Master text styles A. Sterol-dependent regulation of gene • Second level • Third level expression • Fourth level • Fifth level B. Sterol-accelerated enzyme degradation C. Sterol-independent phosphorylation/dephosphorylation D. Hormonal regulation E. Inhibition by drugs

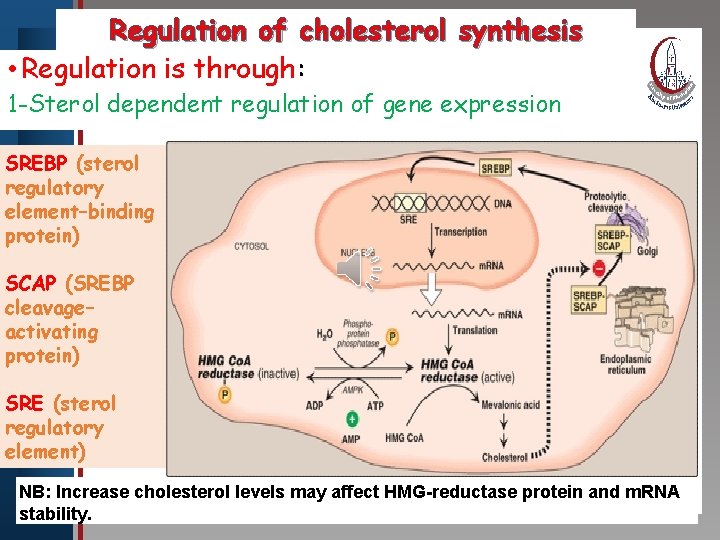
Regulation of cholesterol synthesis • Regulation is through: Click to edit Master title style 1 -Sterol dependent regulation of gene expression • Edit Master text styles SREBP (sterol • Second level regulatory • Third level element–binding • Fourth level protein) • Fifth level SCAP (SREBP cleavage– activating protein) SRE (sterol regulatory element) NB: Increase cholesterol levels may affect HMG-reductase protein and m. RNA stability.

Click to edit Master title style • Edit Master text styles • Second level 2 -Sterol-accelerated enzyme degradation: When sterols are high, HMG Co. A reductase binds to insig proteins targeting it for degradation. • Third level • Fourth level • Fifth level

3 -Sterol independent Click to edit Master title style phosphorylation /dephos-phorylation (Covalent modification). The enzyme reductase is • Edit Master text HMG-Co. A styles • Second level phosphorylated (inactive) by AMPK( • Third level adenosine monophosphate (AMP) • Fourth level • Fifth level activated protein kinase which itself is activated by phosphorylation) and is dephosphorylated (active) by a phosphatase. Cholesterol synthesis is like fatty acid synthesis , is decreased when ATP availability is decreased.

4 -Click Hormonal regulation: Increase to edit Master title style in insulin and thyroxin favors up • Edit Master text styles regulation of the gene • Second level • Third levelof HMG Co. A, while expression glucagon and glucocorticoids have the opposite effects (Insulin stimulates phosphatase enzyme causing its activation) , while glucagon and epinephrine are vice versa. • Fourth level • Fifth level

5 -Drugs inhibition: Statin Click to edit Master title style drugs (lovastatin, rosuvastatin, and • simvastatin) Edit Master text styles are reversible, • Second level competitive inhibitors of HMG • Third level Co. A reductase. They decrease plasma cholesterol levels in patients with hypercholesterolemia. Also they regulate ACAT that changes excess free intracellular cholesterol to esters. • Fourth level • Fifth level

Remember Click to edit Master title style -Regulation • Edit Master text stylesof intracellular • Second level cholesterol is by LDLreceptors mediated uptake and increase HDL mediated reverse transport due to high influx of cholesterol inside the cells. • Third level • Fourth level • Fifth level

Click to edit Master title style • Edit Master text styles • Second level V. Degradation of Cholesterol • Third level • Fourth level • Fifth level

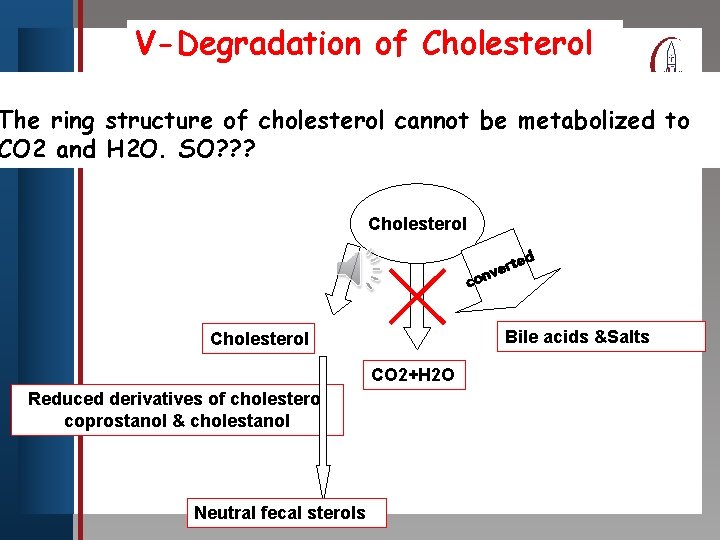
V-Degradation of Cholesterol Click to edit Master title style The ring structure of cholesterol cannot be metabolized to CO 2 • and SO? ? ? Edit. H 2 O. Master text styles • Second level • Third level • Fourth level • Fifth level Cholesterol ed t r e onv c Bile acids &Salts Cholesterol CO 2+H 2 O Reduced derivatives of cholesterol coprostanol & cholestanol Neutral fecal sterols

Click to edit Master title style VI. Bile • Edit Master text. Acids styles • and Bile Salts Second level A. Structure of the bile acids • Third level B. level Synthesis of bile acids • Fourth level C. • Fifth Synthesis of bile salts D. Action of intestinal flora on bile salts E. Enterohepatic circulation F. Bile salt deficiency: cholelithiasis

Bile Click to edit Master title style is a watery mixture formed • Second level by • Third the to be level liver cells stored in gall bladder when not immediately needed for digestion; when needed it directly can pass directly from the liver into the duodenum through the common bile duct. • Edit Master text styles • Fourth level • Fifth level

Click to edit Master title style Remember Bile = • Edit Master text styles • Second level • Third level • Fourth level Bile acids* • Fifth level Bile salt (conjugated bile acid)* Bile pigments (bilirubin glucuronides) Lecithin =Phosphatidylcholine)* Cholesterol *needed for solubilisation

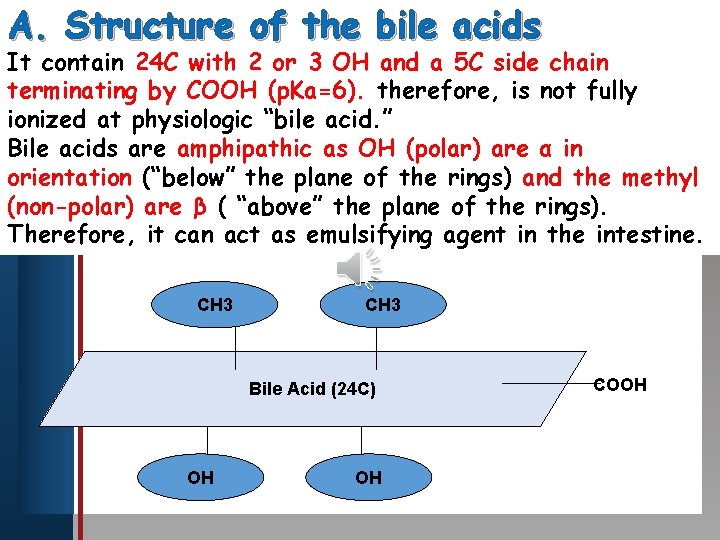
A. Structure of the bile acids It contain 24 C with 2 or 3 OH and a 5 C side chain Click toby edit Master title therefore, style terminating COOH (p. Ka=6). is not fully ionized at physiologic “bile acid. ” Bile • acids are amphipathic as OH (polar) are α in Edit Master text styles orientation (“below” the plane of the rings) and the methyl • Second level Third level (non-polar) • are β ( “above” the plane of the rings). Fourth level Therefore, it • can act as emulsifying agent in the intestine. • Fifth level CH 3 Bile Acid (24 C) OH OH COOH

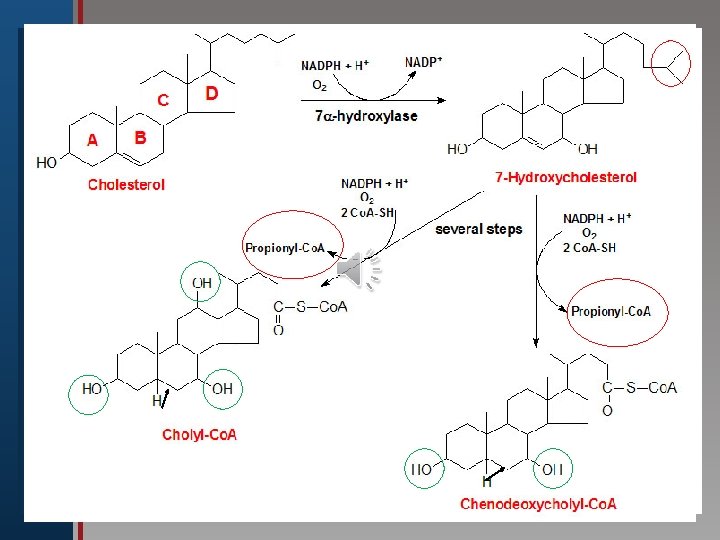
Click to edit Master title style B-Synthesis of Bile Acids: 1 ry bile acids (cholic & chenodeoxycholic • Edit Master text styles acid): • Second level • Third level The first and rate limiting step in their • Fourth level synthesis is • Fifth the level introduction of hydroxyl group at C 7 by 7α–hydroxylase enzyme (monooxygenase ), which requires oxygen, cytochrome P 450 and NADPH. The enzyme is subject to feed back inhibition by bile salts.

Click to edit Master title style • Edit Master text styles • Second level • Third level • Fourth level • Fifth level

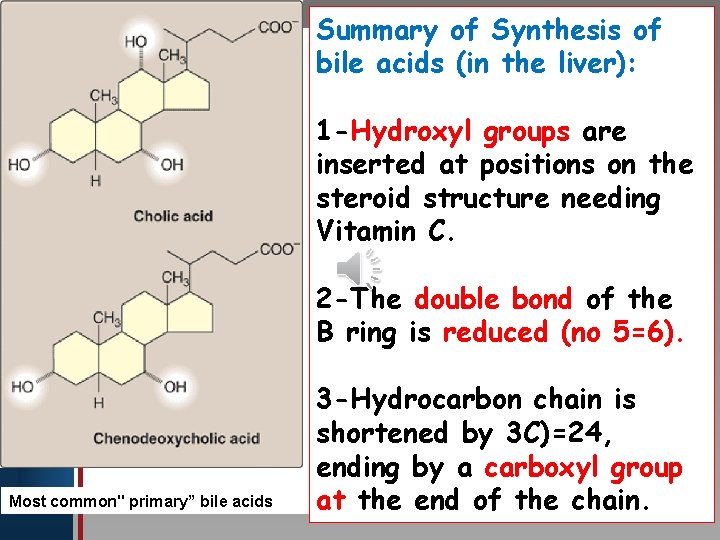
Summary of Synthesis of bile acids (in the liver): Click to edit Master title style • Edit Master text styles • Second level • Third level • Fourth level • Fifth level 1 -Hydroxyl groups are inserted at positions on the steroid structure needing Vitamin C. 2 -The double bond of the B ring is reduced (no 5=6). Most common" primary” bile acids 3 -Hydrocarbon chain is shortened by 3 C)=24, ending by a carboxyl group at the end of the chain.

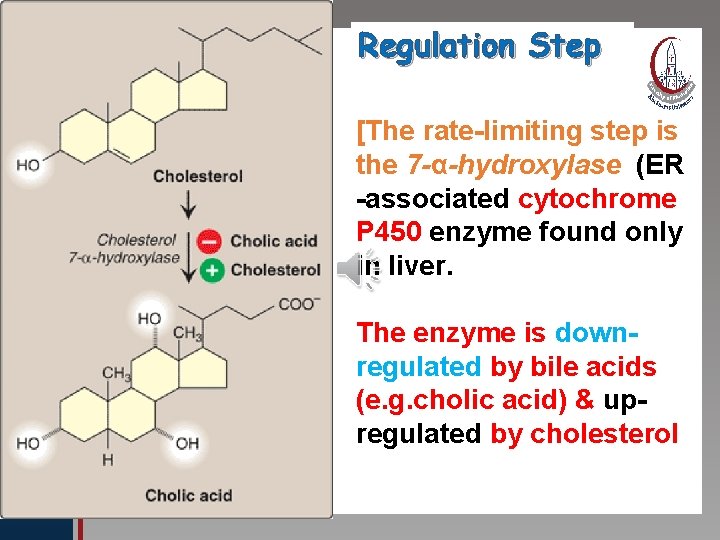
Regulation Step Click to edit Master title style • Edit Master text styles • Second level • Third level • Fourth level • Fifth level [The rate-limiting step is the 7 -α-hydroxylase (ER -associated cytochrome P 450 enzyme found only in liver. The enzyme is downregulated by bile acids (e. g. cholic acid) & upregulated by cholesterol

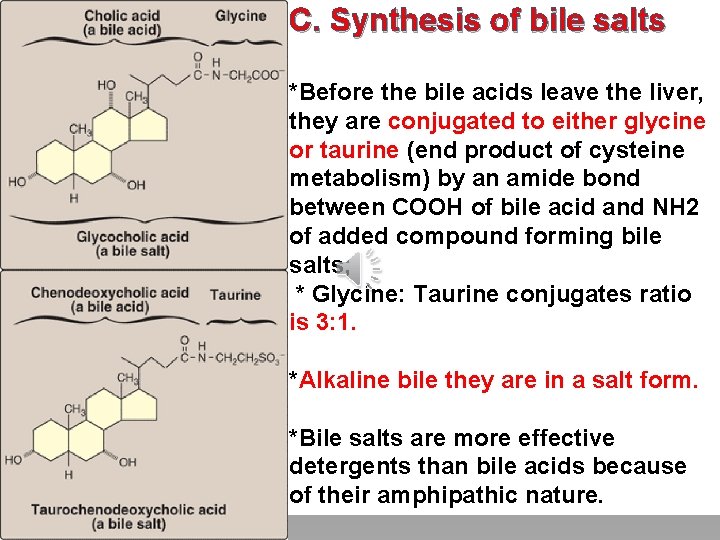
C. Synthesis of bile salts Click to edit Master title style *Before the bile acids leave the liver, they are conjugated to either glycine • Edit Master text styles or taurine (end product of cysteine metabolism) by an amide bond • Second level • Third level between COOH of bile acid and NH 2 • Fourth level • Fifth level of added compound forming bile salts; * Glycine: Taurine conjugates ratio is 3: 1. *Alkaline bile they are in a salt form. *Bile salts are more effective detergents than bile acids because of their amphipathic nature.

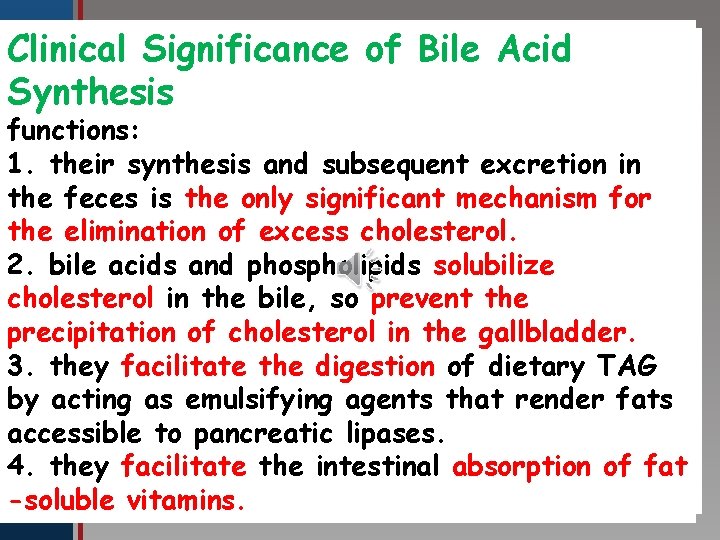
Clinical Significance of Bile Acid Click to edit Master title style Synthesis functions: • Edit Master text stylesand subsequent excretion in 1. their synthesis • Second level the feces • Third is level the only significant mechanism for • Fourth level the elimination of excess cholesterol. • Fifth level 2. bile acids and phospholipids solubilize cholesterol in the bile, so prevent the precipitation of cholesterol in the gallbladder. 3. they facilitate the digestion of dietary TAG by acting as emulsifying agents that render fats accessible to pancreatic lipases. 4. they facilitate the intestinal absorption of fat -soluble vitamins.

D. Action of intestinal flora on Click to edit Master style biletitle salts • 1 Edit Can Masterremove text styles glycine and taurine from • Second level bile salts, regenerating bile acids. • Third level • Fourth level 2 - Convert some of the primary bile • Fifth level acids into “secondary” bile acids by removing a hydroxyl group (7), producing deoxycholic acid from cholic acid And lithocholic acid from chenodeoxycholic acid

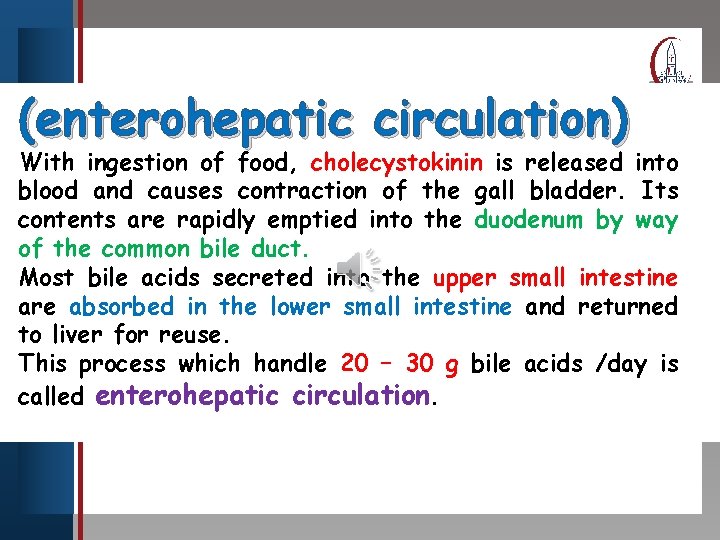
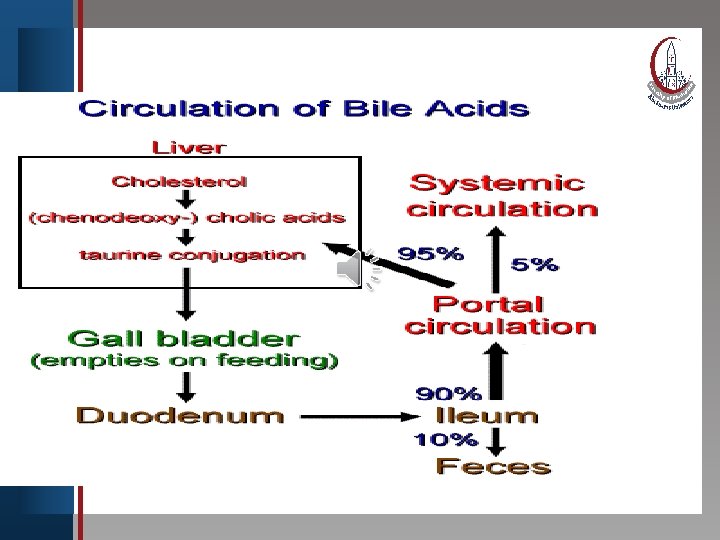
Click to edit Master title style (enterohepatic circulation) • Edit Master text With ingestion ofstyles food, cholecystokinin is released into Second level blood • and causes contraction of the gall bladder. Its • Third level contents are • rapidly Fourth level emptied into the duodenum by way Fifth level of the common • bile duct. Most bile acids secreted into the upper small intestine are absorbed in the lower small intestine and returned to liver for reuse. This process which handle 20 – 30 g bile acids /day is called enterohepatic circulation.

Click to edit Master title style Daily elimination of bile acids in feces • Edit Master text styles amounts to 0. 5 g/day or less. The bile • Second level enterohepatic circulation acids • in. Third the • Fourth level • Fifth level during digestion of each circulates twice meal. NB. When bile acid binding resin is given, the re-absorption of bile is inhibited , so more cholesterol is converted to bile acids decreasing intracellular cholesterol.

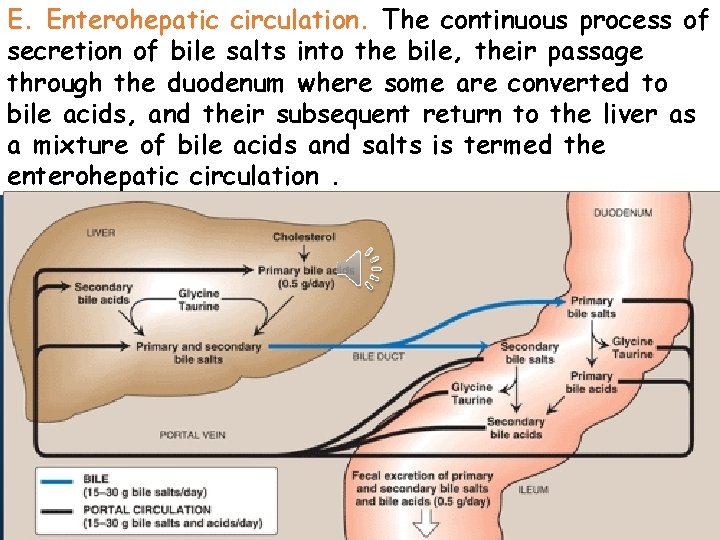
E. Enterohepatic circulation. The continuous process of secretion of bile salts into the bile, their passage through duodenum some are converted to Clickthe to edit Masterwhere title style bile acids, and their subsequent return to the liver as a mixture of bile acids and salts is termed the • Edit Master text styles enterohepatic circulation. • Second level • Third level • Fourth level • Fifth level

Click to edit Master title style • Edit Master text styles • Second level • Third level • Fourth level • Fifth level

Bile salts deficiency- Cholelithiasis https: //youtu. be/2 FY 0 b. ZFg. MCA Click to edit Master title https: //youtu. be/2 d. Xb. H 3 du 4 Ds style Cholelithiasis Is the presence of one or more calculi • Edit Master text styles (gallstones) in the gal • Second level lbladder. • Third level Different types exist, • Fourth level and they are categorized by their • Fifth level primary composition. Cholesterol stones are the most common. Normally, bile acids and salts, lecithin, and phospholipids help to maintain cholesterol solubility in bile. When the ratio of cholesterol to bile acids or phospholipids is increased, bile becomes supersaturated with cholesterol; it crystallizes and forms a nucleus for stone formation.

Click to edit Master title style Laparoscopic surgical cholecystectomy is • Edit Master text styles currently • Second level the treatment of choice. • Third level However, for patients who are unable to undergo surgery, administration of (bile salts) chenodeoxycholic acid to supplement the body's supply of bile acids results in a gradual dissolution of the gallstones. • Fourth level • Fifth level

Gall stone is mainly due to the decrease of bile acids in the bile, resulting from Click to 1. edit Master title style bile remains in GB Gall bladder stasis: long periods (super-saturation in DM) • Edit Master text styles • Second level 2. Biliary tract obstruction • Third level • Fourth level • Fifth level 3. Severe hepatic dysfunction (decreased bile synthesis). (Cirrhosis 10 Xincrease for risk of gallstones), perhaps also due to impaired GB contraction or high estrogen levels occurring with cirrhosis. 4. drugs as fibrates (derivative of fibric acid used to lower serum TAG but increasing biliary cholesterol excretion).

Other Risk Factors for Cholelethiasis: Click to edit Master title style 1. Obesity : Cholesterol gallstones due to enhanced cholesterol synthesis and • Edit Master text styles secretion. • Second level • Thirdinactivity: level 2. Physical Gall stones could be • Fourth level minutes of daily aerobic prevented by • Fifth 30 exercise. 3. Elevated estrogen and progesterone: As (pregnancy, oral contraceptives or hormone therapy) they induce changes in the biliary system stones. 4. Increasing age: Gallstones increases over 40 y.

Click to edit Master title style • Edit Master text styles • Second level • Third level • Fourth level • Fifth level