Clay Mineralogy Clay is a particle SIZE Predominant




























- Slides: 51

Clay Mineralogy

• Clay is a particle SIZE • Predominant make-up is SECONDARY minerals

Minerals can be crystalline or amorphous. Example: Si. O 2 crystalline QUARTZ (Si. O 2) : resistant to weathering Amorphous silica (Si. O 2) : 10 x more soluble

1. Silicate Clays (crystalline) 2. Sesquioxide/oxidic clays 3. Amorphous clays (non-crystalline)

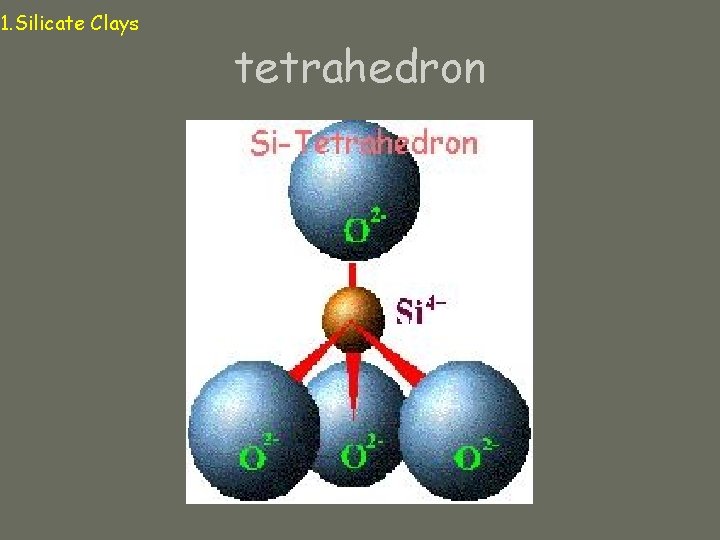
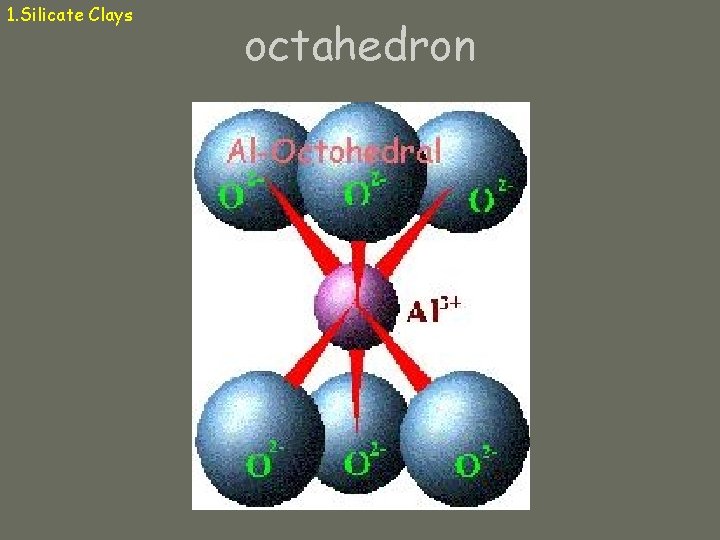
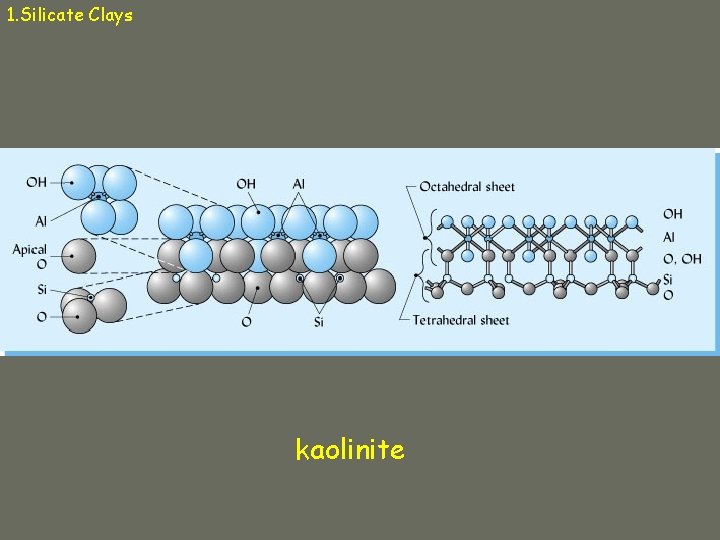
1. Silicate Clays (aluminosilicates) Micelle: particle of silicate clay Composed of tetrahedral and octahedral “sandwiches” Tetrahedron: central cation (Si+4, Al+3) surrounded by 4 oxygens Octahedron: central cation (Al+3, Fe+2, Mg+2) surrounded by 6 oxygens (or hydroxyls)

1. Silicate Clays tetrahedron

1. Silicate Clays

1. Silicate Clays octahedron

1. Silicate Clays

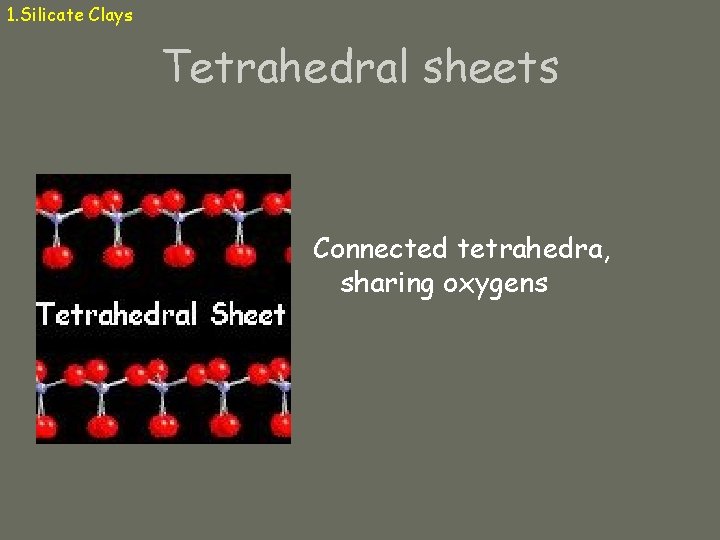
1. Silicate Clays Tetrahedral sheets Connected tetrahedra, sharing oxygens

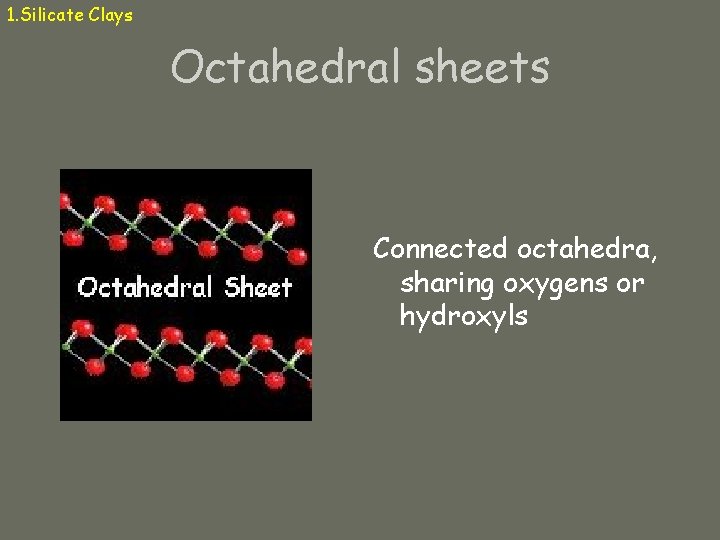
1. Silicate Clays Octahedral sheets Connected octahedra, sharing oxygens or hydroxyls

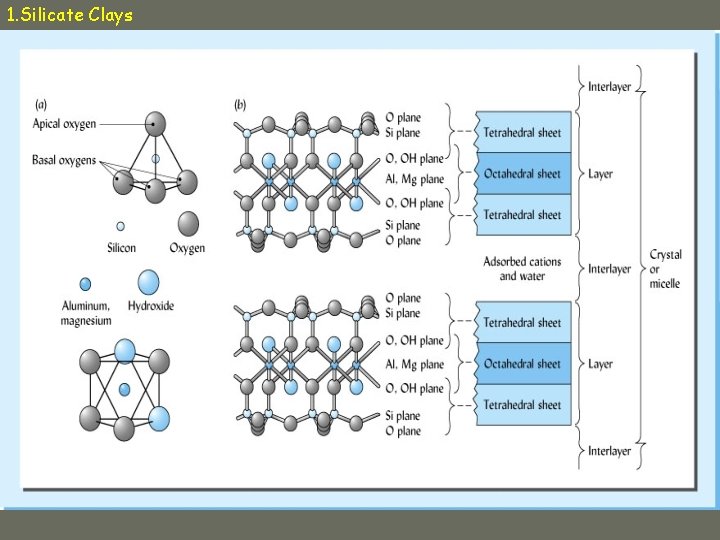
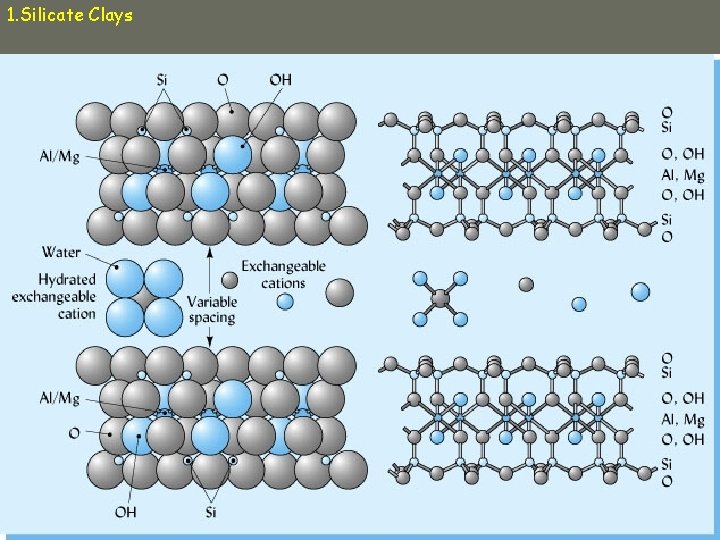
1. Silicate Clays • 1000 s of tetrahedra and octahedra connect in clay minerals to give: – Planes of Si, Al, Mg – Planes of Oxygen, hydroxyl groups • Sheets combine to form layers • Layers are separated by interlayer space – Water, adsorbed cations

1. Silicate Clays

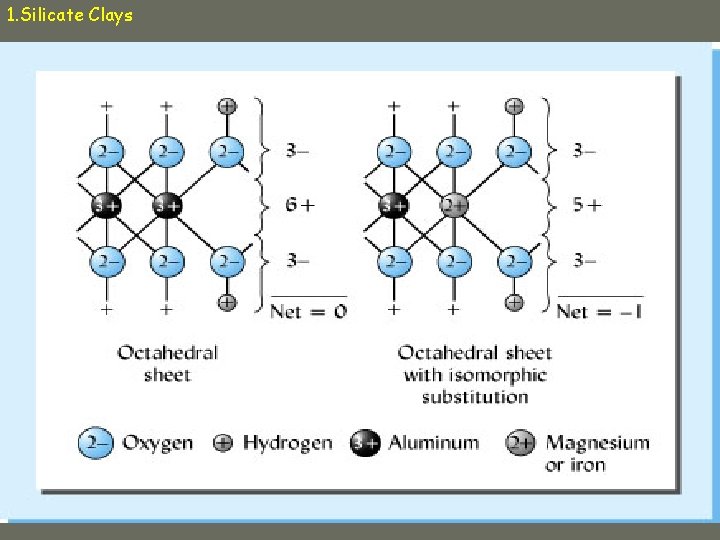
1. Silicate Clays Isomorphous substitution Lower charge cations replace higher charge cations as central cation – E. g. , Mg+2 replaces Al+3 • leaves net negative charge

1. Silicate Clays

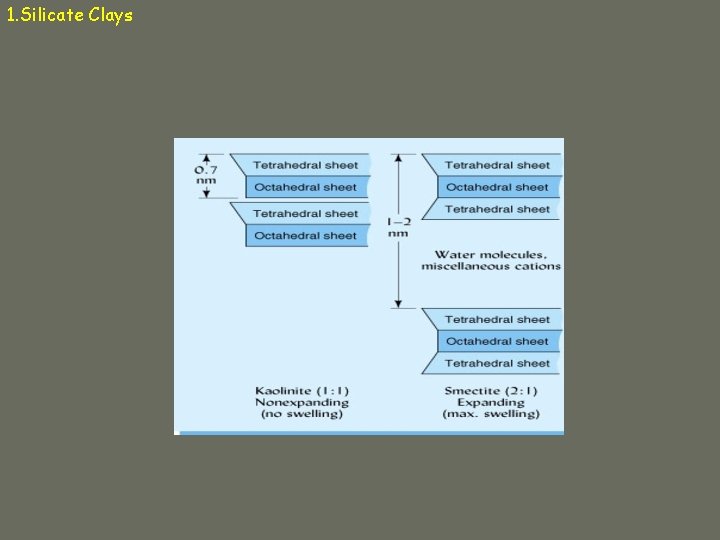
1. Silicate Clays Different types of silicate clays are composed of sandwiches (combinations) of layers with various substances in their interlayer space. 2: 1 two tetrahedral sheets to one octahedral sheet 1: 1 one tetrahedron sheet to one octahedral sheet

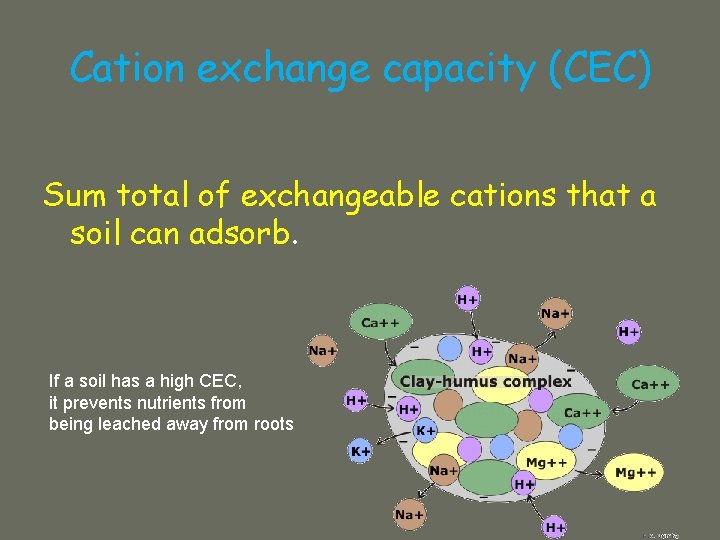
Cation exchange capacity (CEC) Sum total of exchangeable cations that a soil can adsorb. If a soil has a high CEC, it prevents nutrients from being leached away from roots

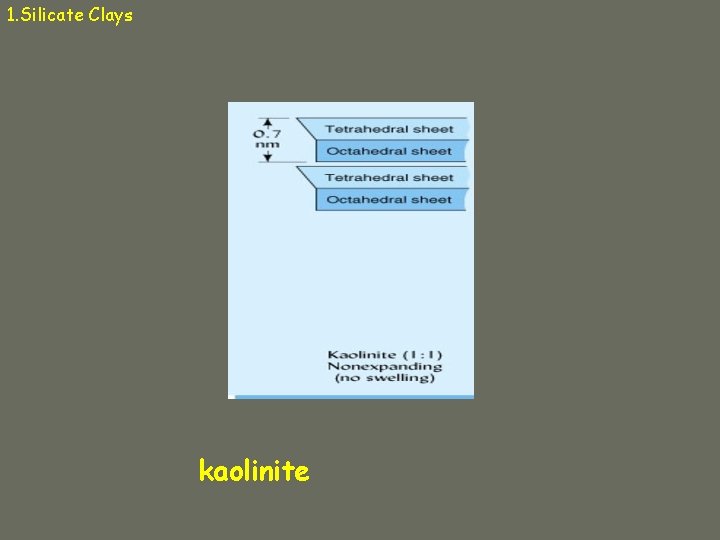
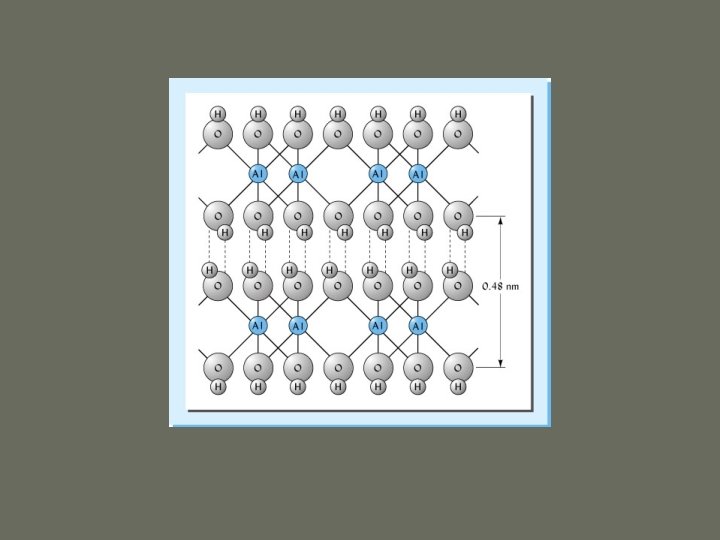
1. Silicate Clays a. Kaolinite ü 1: 1 ü Hydrogen bonds in interlayer space üstrong ü Nonexpandable ü Low CEC ü Particles can grow very large (0. 2 – 2 µm) ü Effective surface area = 10 – 30 m 2/g üExternal surface only

1. Silicate Clays kaolinite

1. Silicate Clays kaolinite

1. Silicate Clays Kaolinite ü good road base ü good foundation ü good for pottery; China clay (porcelain) ü easy to cultivate, but need manure or fertilizer ü Dominant clay mineral in highly weathered soils

Kaolin mine, Bulgaria

Kaolinite mine, MN (MN River Valley)

Click here, Pat

1. Silicate Clays kaolinite


1. Silicate Clays b. Smectite ü 2: 1 ü Weathering product ü Always negative due to isomorphous substitution ü Layers weakly held together by weak O-O bonds or cation-O bonds ü Cations adsorbed in interlayer space ü Expandable ü High CEC

1. Silicate Clays

1. Silicate Clays

1. Silicate Clays smectite ü Interlayer cations hold layers together In dry soils, bonding force is strong and hard clods form; deep cracks In wet soils, water is drawn into interlayer space and clay swells. ü Montmorillonite ü Vertisols ü Dominant clay mineral of most MN soils

1. Silicate Clays smectite ü High effective surface area = 650 – 800 m 2/g ü Internal surface area >> external ü Particles small ü Most expandable of all clays


Click here, Pat

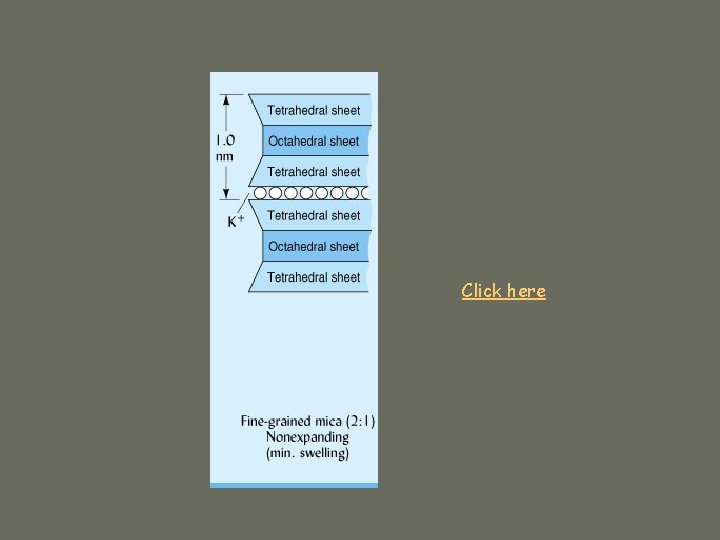
1. Silicate Clays c. Fine-grained micas ü 2: 1 ü As mica crystallizes from magma: ü Isomorphous substitution of Al+3 for Si+4 in tetrahedra ü high net negative charge ü K+ ions in interlayer space ü Strongly binds layers ü Non-expandable ü Illite ü Surface area 70 -175 m 2/g

Click here

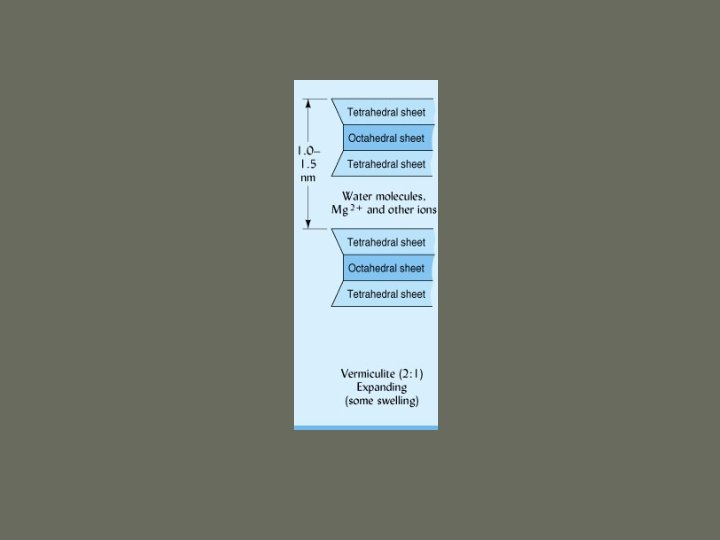
1. Silicate Clays d. Vermiculite ü 2: 1 ü Forms from alteration of mica üWeathering removes some K+ ions üReplaced by hydrated cations in interlayer space ü Water molecules and cations bridge layers, so not as expandable as smectites

1. Silicate Clays ü Still have very high net negative charge ü High CEC (highest of all clays) ü Expandable ü Octahedral ions are Al, Mg, Fe ü Surface area 600 – 800 m 2/g üInternal >> external


Click here

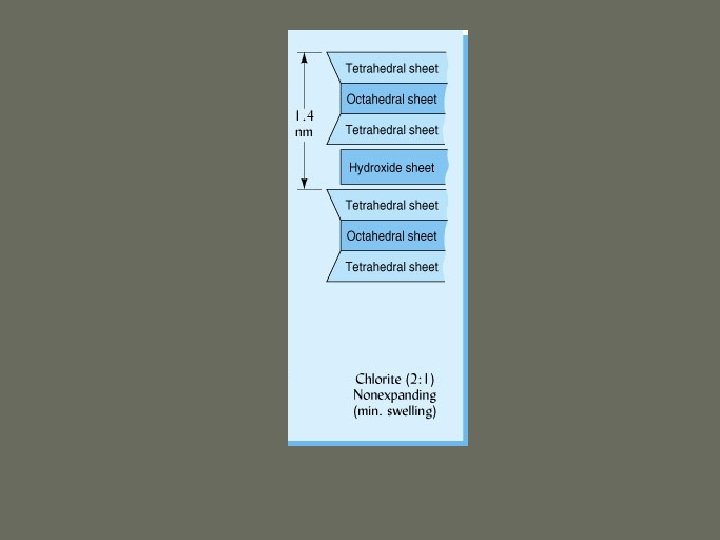
1. Silicate Clays e. Chlorite ü 2: 1 ü Central cations in octahedral sheets is Fe or Mg ü Interlayer space occupied by a stable, positively charged octahedral sheet ü Non-expandable ü 70 -100 m 2/g surface area


Click here

2. Sesquioxides / Oxidic Clays ü Ultimate weathering products ü Ultisols and Oxisols ü Very stable; persist indefinitely ü Yellow, red, brown ü Fe or Al as central cations ü Lack negative charge ü Don’t retain adsorbed cations ü Non-expandable ü Low CEC

Ultisol profile

ü In heavily leached soils, sheets decompose into component Si tet. and Al oct. üAl oct. often weather into gibbsite Al(OH)3


3. Amorphous (non-crystalline) ü silicates ü Allophane and imogolite ü Common in volcanic ash ü High internal negative charge ü High CEC ü High water-holding capacity ü Surface area 100 – 1000 m 2/g

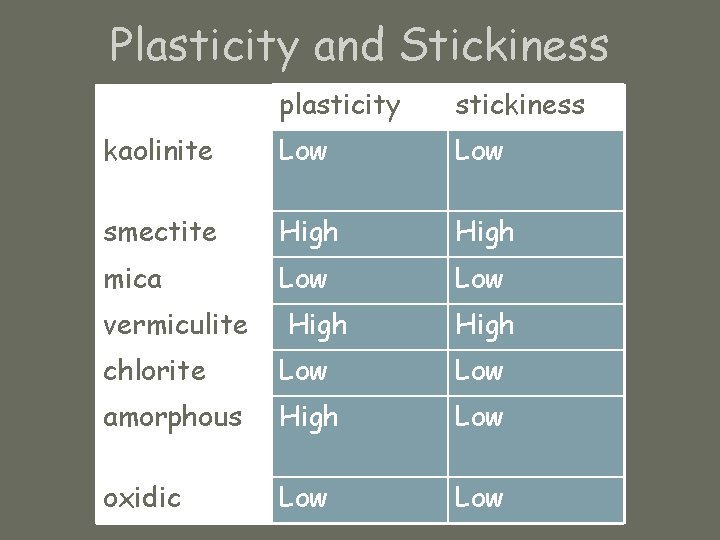
Plasticity and Stickiness plasticity stickiness kaolinite Low smectite High mica Low vermiculite High chlorite Low amorphous High Low oxidic Low

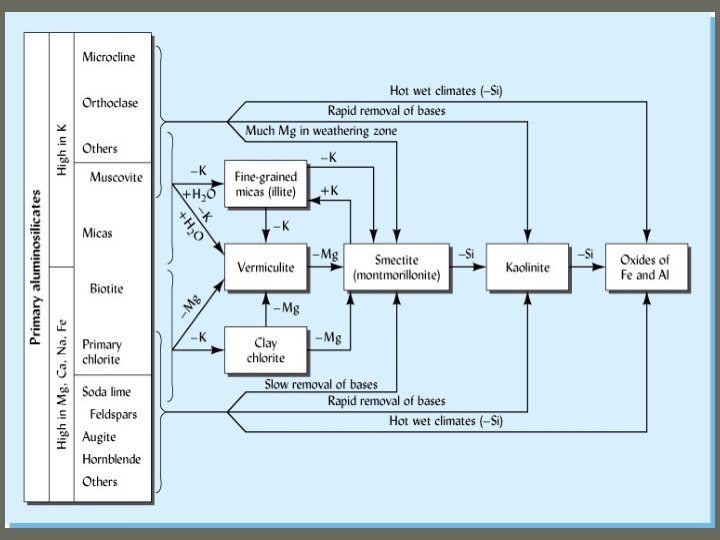
What determines clay minerals in a given soil? ü Usually a mixture ü Climate ü Parent material ü Degree of weathering


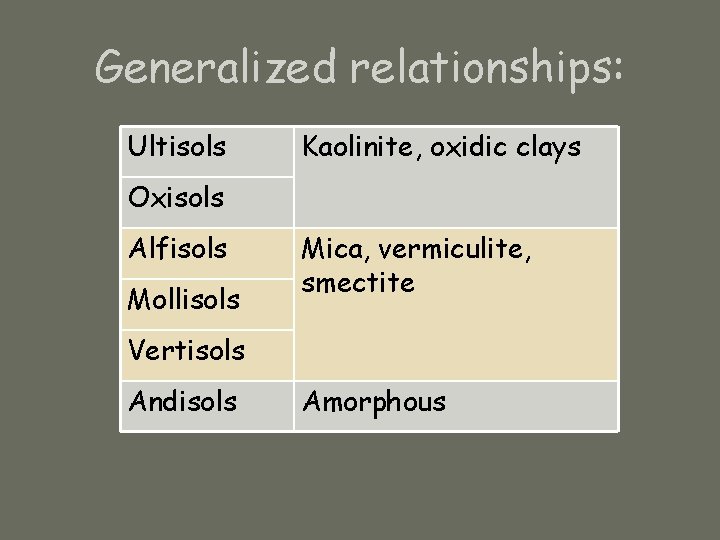
Generalized relationships: Ultisols Kaolinite, oxidic clays Oxisols Alfisols Mollisols Mica, vermiculite, smectite Vertisols Andisols Amorphous
 Types of extinction in minerals
Types of extinction in minerals Kyanite formula
Kyanite formula What is this
What is this Mineralogy lab
Mineralogy lab Optical mineralogy
Optical mineralogy Melatope mineralogy
Melatope mineralogy Isochromes
Isochromes Uniaxial and biaxial minerals
Uniaxial and biaxial minerals Mineralogy
Mineralogy Andreasen pipette principle
Andreasen pipette principle Horiba la-960 instruction manual
Horiba la-960 instruction manual Soil particle size classification
Soil particle size classification Identify the cell
Identify the cell What is atp pc
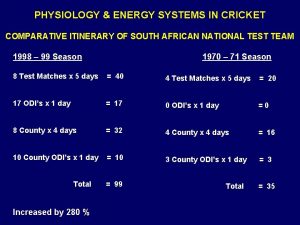
What is atp pc Predominant energy system
Predominant energy system Predominant church affiliation by county 2000 frq
Predominant church affiliation by county 2000 frq Predominant macroconidia and scanty microconidia
Predominant macroconidia and scanty microconidia Pentameter types
Pentameter types What cell
What cell What is predominant energy system
What is predominant energy system What is predominant energy system
What is predominant energy system Lenf nedir
Lenf nedir What is predominant energy system
What is predominant energy system What is predominant energy system
What is predominant energy system Const char *s
Const char *s Agitation method
Agitation method Pythia set
Pythia set Exchange particle
Exchange particle The god particle plugin
The god particle plugin What is a particle
What is a particle Beta particle charge
Beta particle charge Particle theory examples
Particle theory examples Particle model of matter exam questions
Particle model of matter exam questions Beta particle charge
Beta particle charge Axion particle
Axion particle Particle
Particle Erik adli
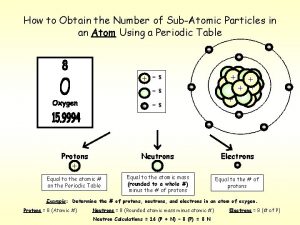
Erik adli The first subatomic particle discovered was the
The first subatomic particle discovered was the Mixture particle diagram
Mixture particle diagram Rest energy of a proton
Rest energy of a proton Phrasal verbs literal and idiomatic
Phrasal verbs literal and idiomatic Pierpaolo palestri
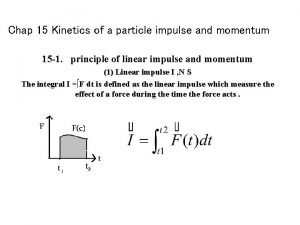
Pierpaolo palestri Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Derived properties of powder
Derived properties of powder Beta particle charge
Beta particle charge Whats the smallest particle of matter
Whats the smallest particle of matter Dynamics of a particle moving in a straight line
Dynamics of a particle moving in a straight line Particle gun
Particle gun Pure substance vs element
Pure substance vs element Particle vs rigid body
Particle vs rigid body A particle limited to the x axis has the wave function
A particle limited to the x axis has the wave function Anatoli bugorski
Anatoli bugorski