Clathrates Clathrates Cagelike frameworks of metals with other



























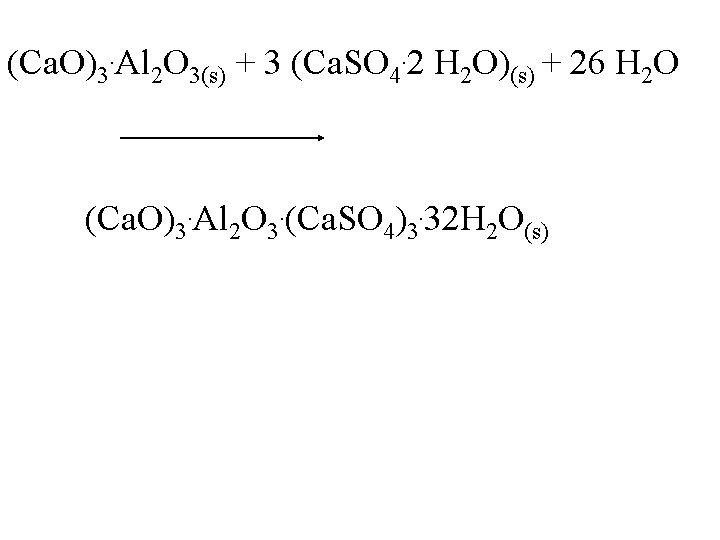

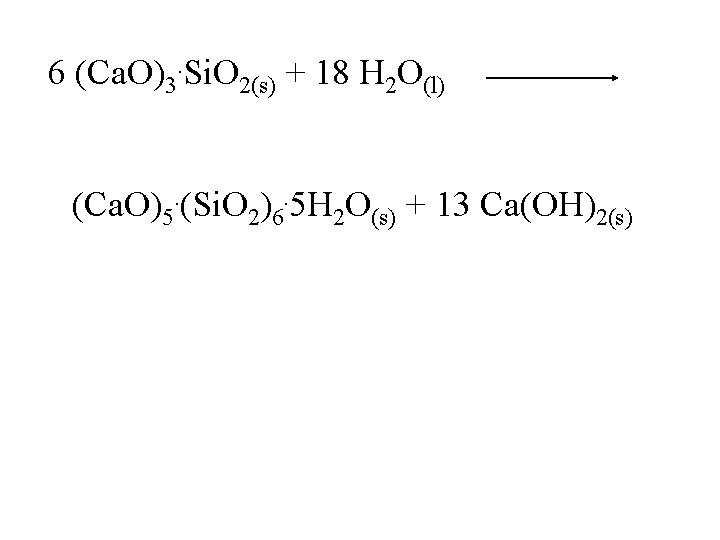
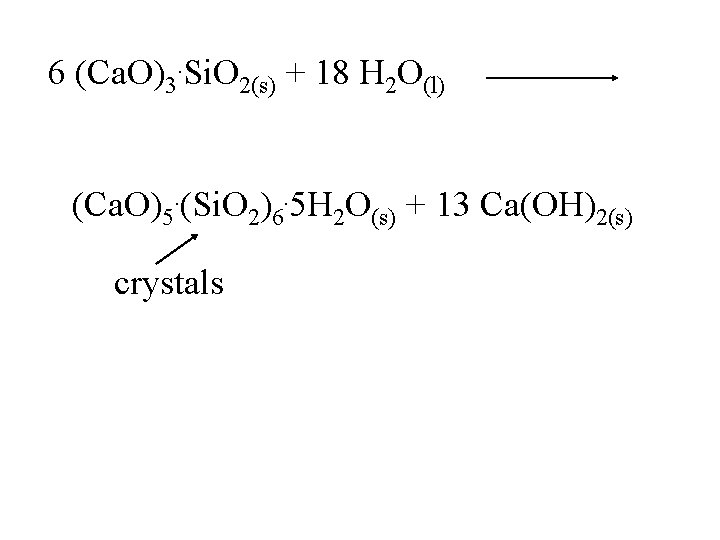

- Slides: 57

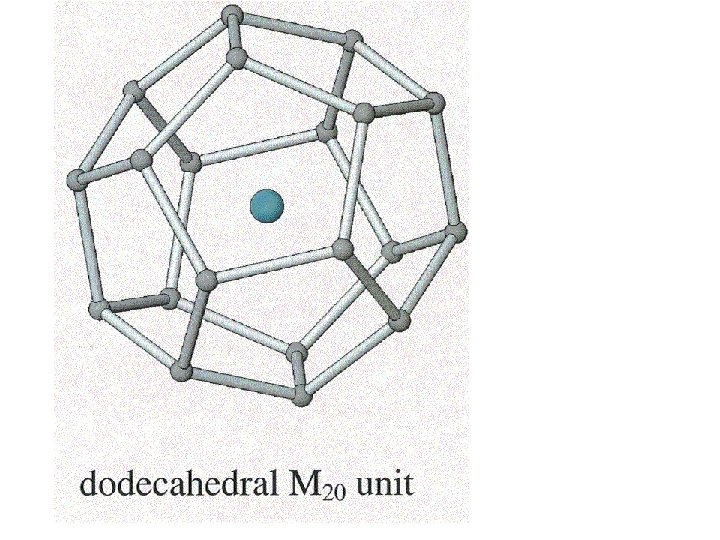
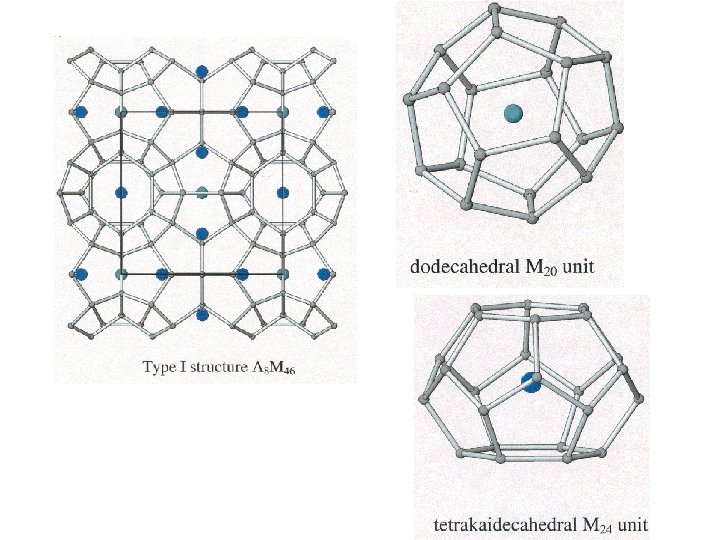
Clathrates

Clathrates Cage-like frameworks of metals with other metals occupying the cavities of the cages.

Clathrates These can be synthesized by mixing finely divided quantities of the metals in the correct proportions and careful heating and cooling.





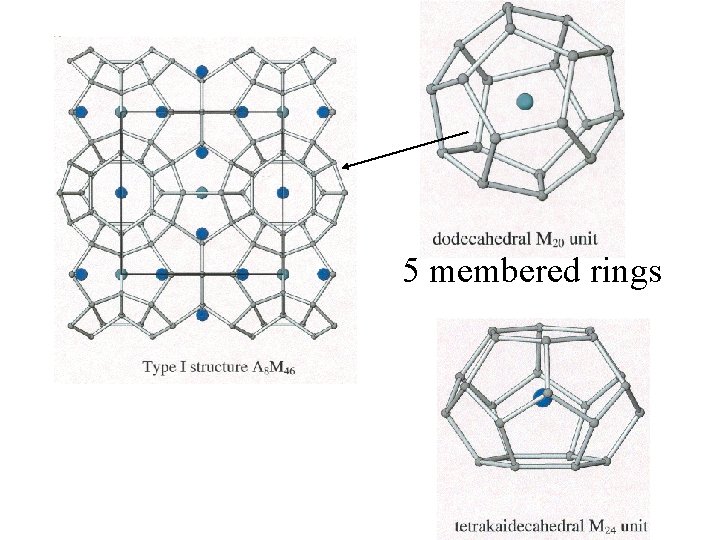
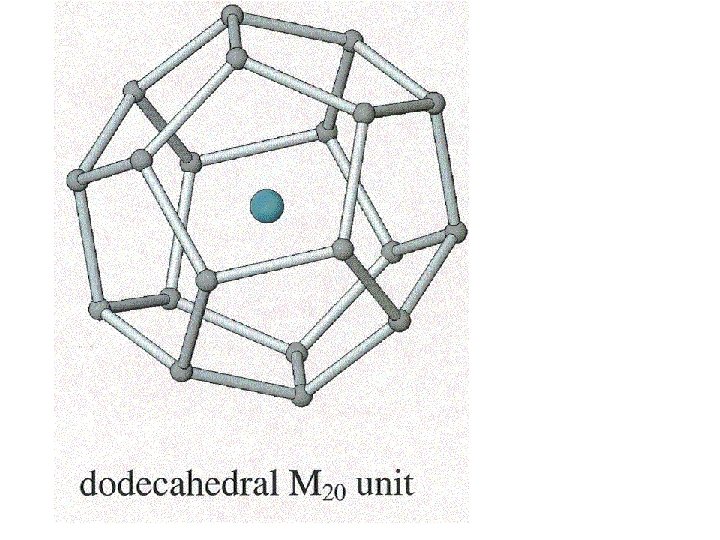
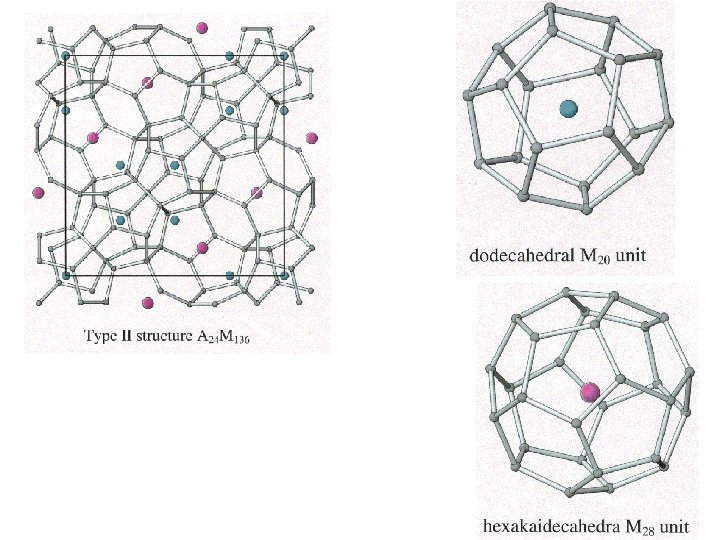
5 membered rings

5 membered rings 5 + 6 membered rings





Clathrates of this type have useful thermal and semiconductor properties.

Clathrates of this type have useful thermal and semiconductor properties. A good semiconductor that has poor thermal conductivity is useful for making a thermo-electric device.

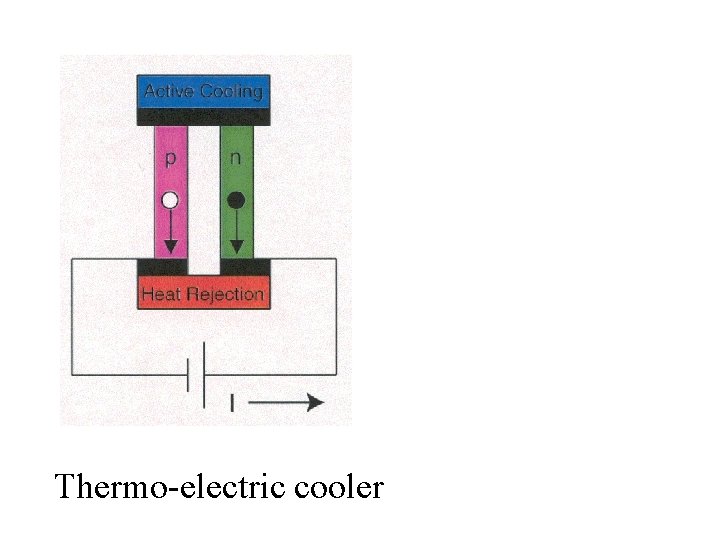
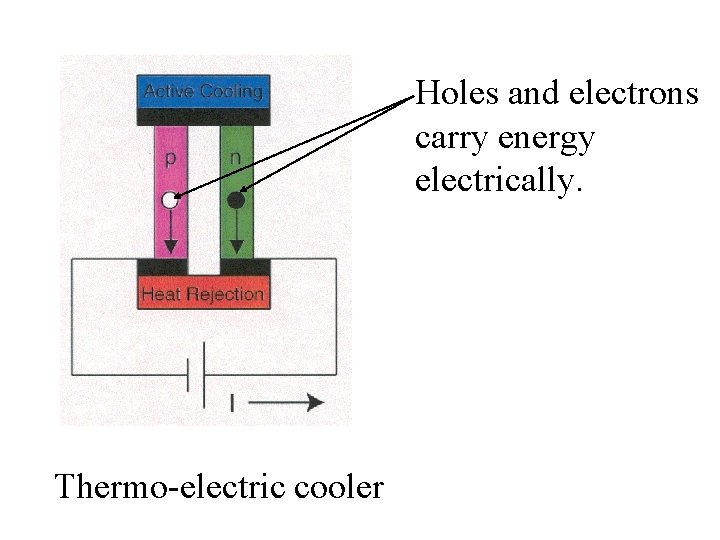
Thermo-electric cooler

Holes and electrons carry energy electrically. Thermo-electric cooler

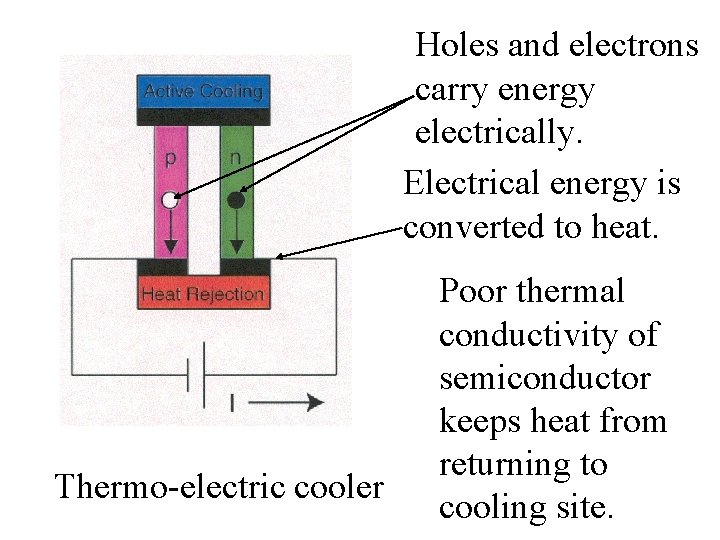
Holes and electrons carry energy electrically. Electrical energy is converted to heat. Thermo-electric cooler

Holes and electrons carry energy electrically. Electrical energy is converted to heat. Thermo-electric cooler Poor thermal conductivity of semiconductor keeps heat from returning to cooling site.

Ceramics and glass

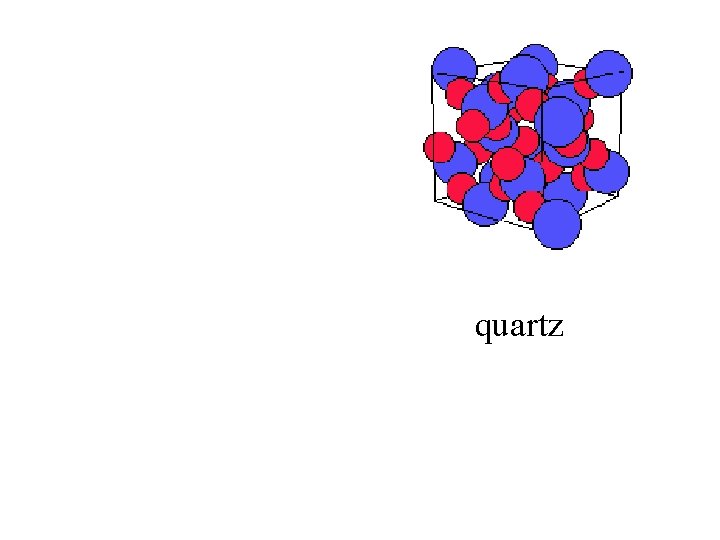
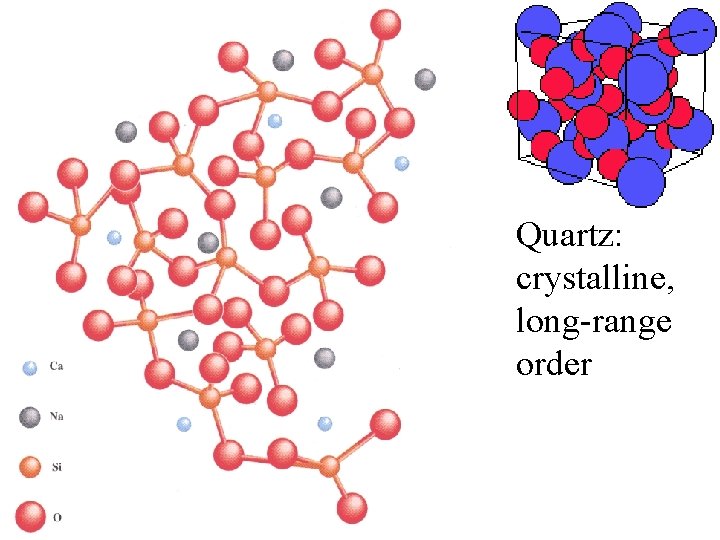
quartz

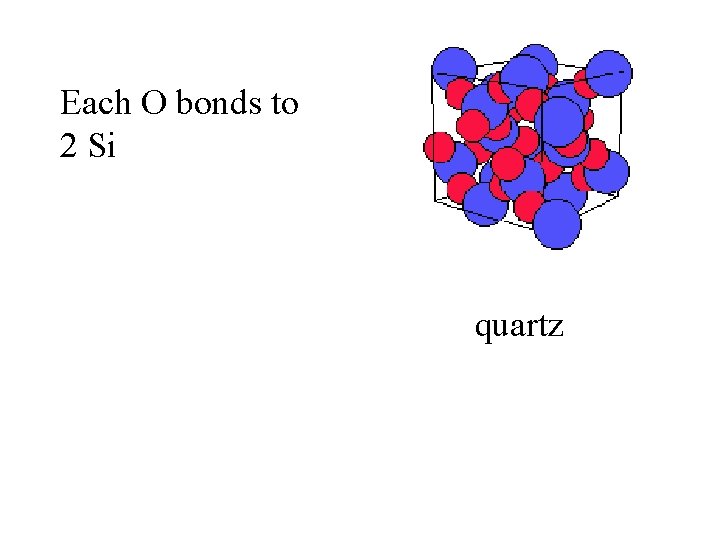
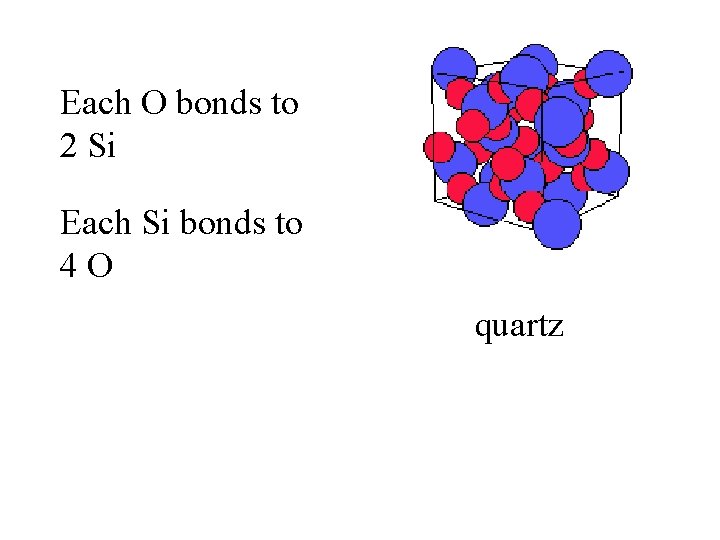
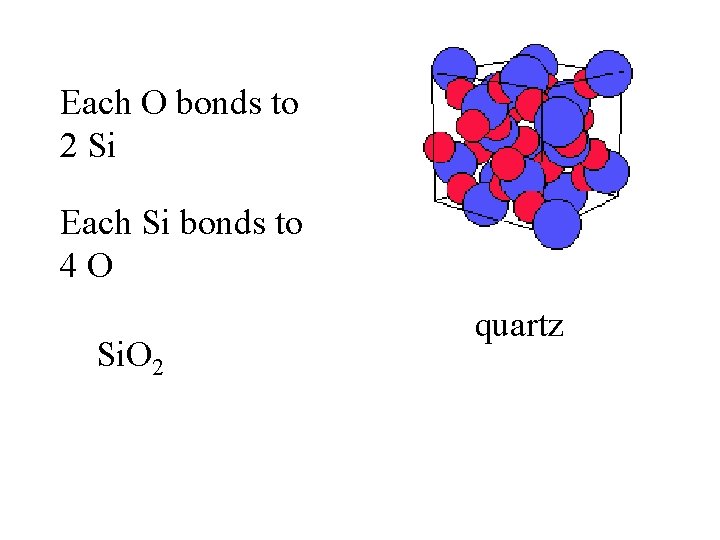
Each O bonds to 2 Si quartz

Each O bonds to 2 Si Each Si bonds to 4 O quartz

Each O bonds to 2 Si Each Si bonds to 4 O Si. O 2 quartz

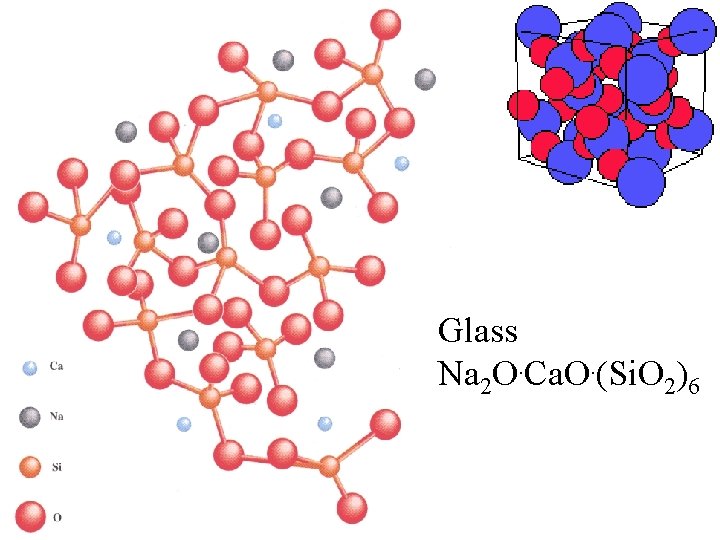
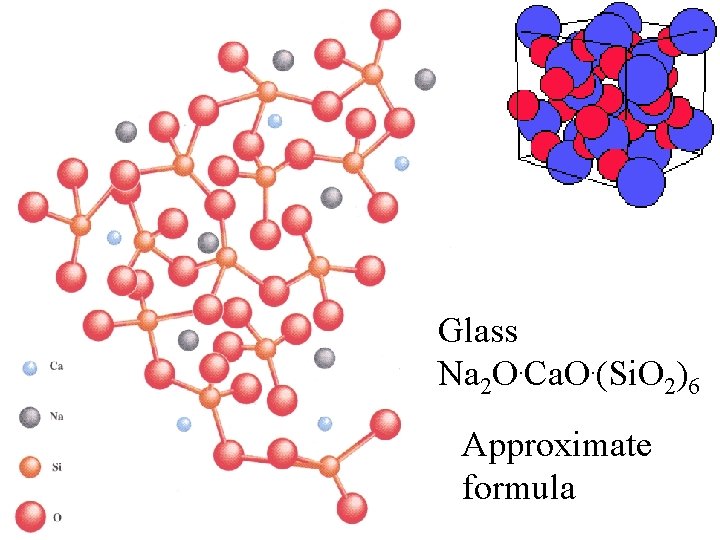
Glass Na 2 O. Ca. O. (Si. O 2)6

Glass Na 2 O. Ca. O. (Si. O 2)6 Approximate formula

Quartz: crystalline, long-range order

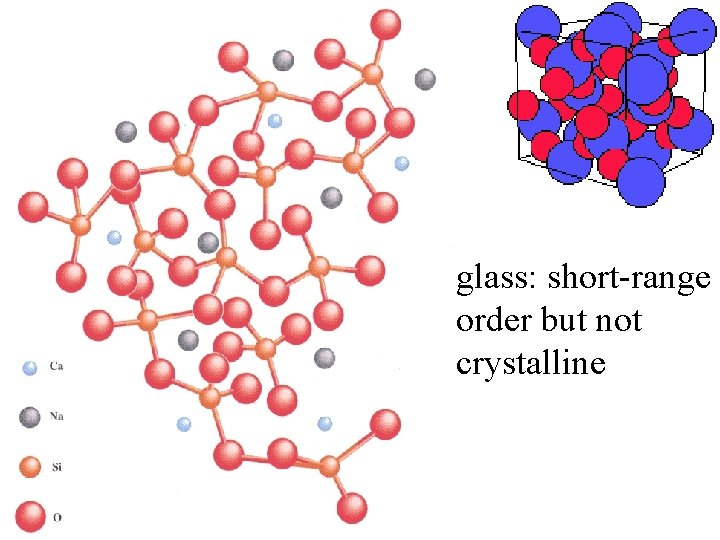
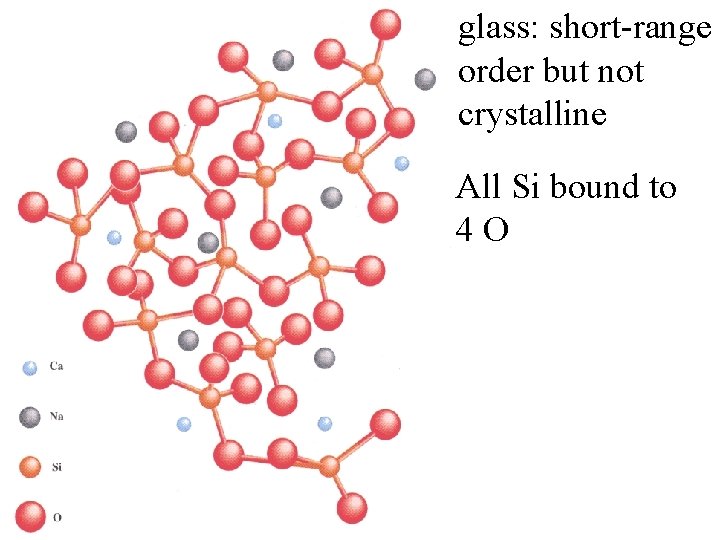
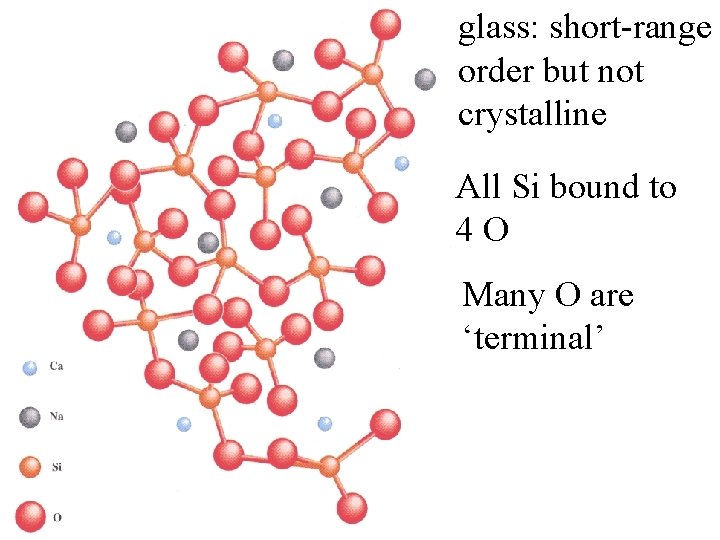
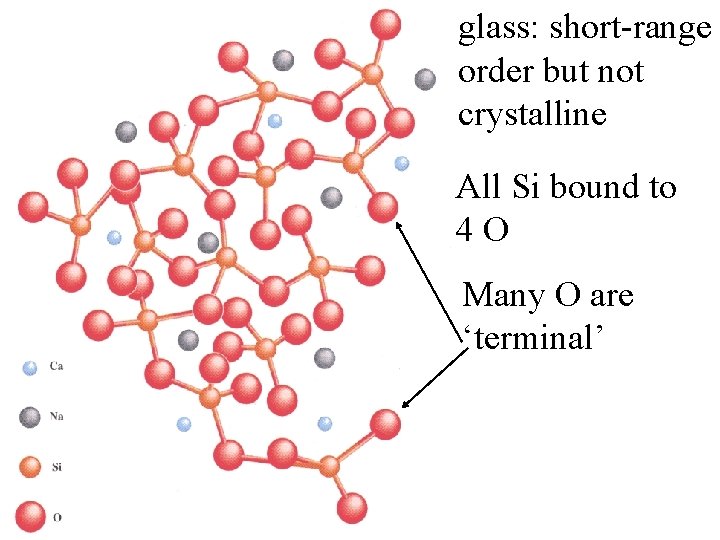
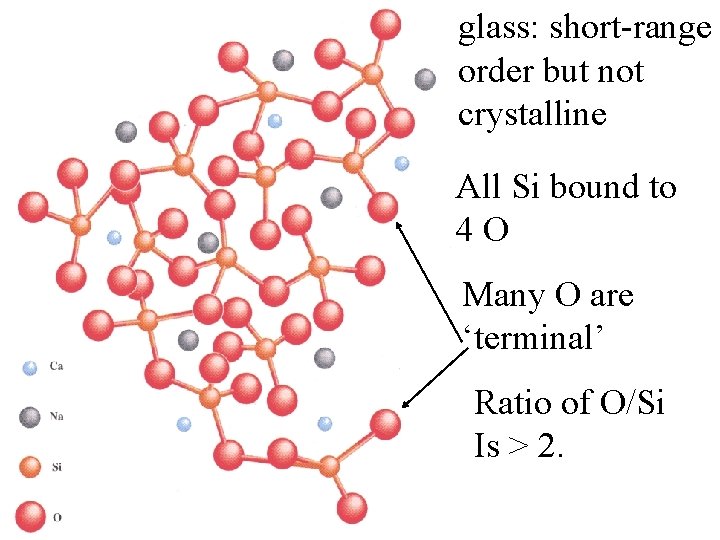
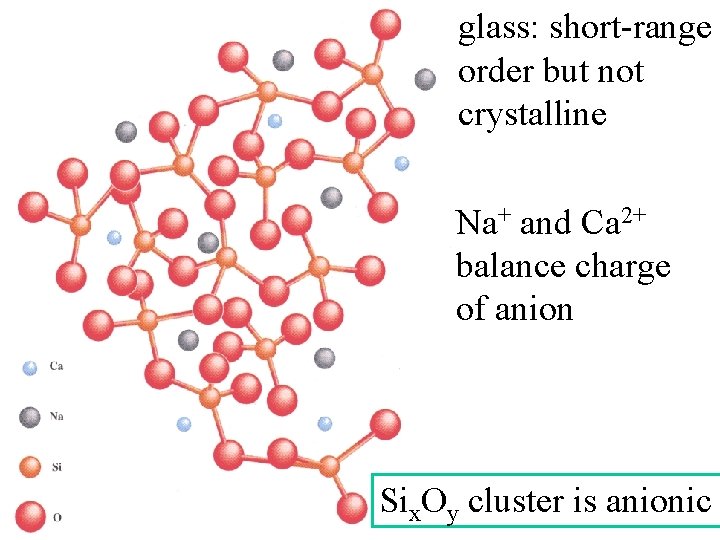
glass: short-range order but not crystalline

glass: short-range order but not crystalline All Si bound to 4 O

glass: short-range order but not crystalline All Si bound to 4 O Many O are ‘terminal’

glass: short-range order but not crystalline All Si bound to 4 O Many O are ‘terminal’

glass: short-range order but not crystalline All Si bound to 4 O Many O are ‘terminal’ Ratio of O/Si Is > 2.

glass: short-range order but not crystalline All Si bound to 4 O Many O are ‘terminal’ Ratio of O/Si Is > 2. Six. Oy cluster is anionic

glass: short-range order but not crystalline Na+ and Ca 2+ balance charge of anion Six. Oy cluster is anionic

Properties of glass vs. quartz.

Properties of glass vs. quartz. Glass has a lower melting point

Properties of glass vs. quartz. Glass has a lower melting point Glass is softer Glass does not crystallize – this makes it easier to shape it as it cools to a solid form.

Special glasses:

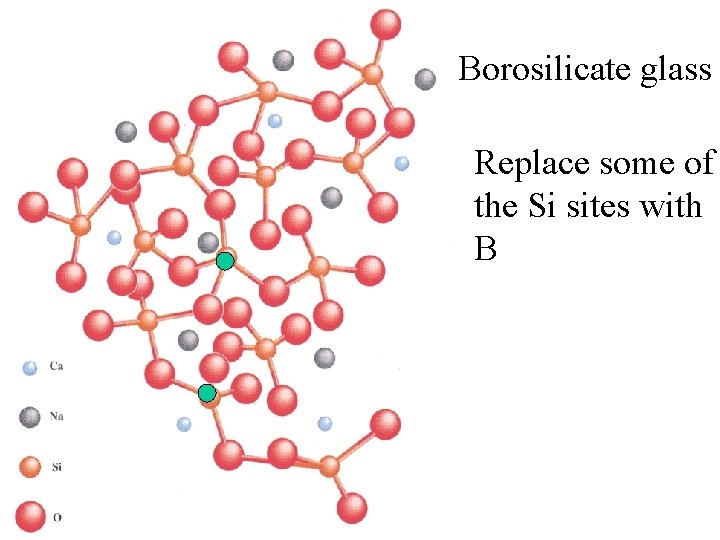
Special glasses: Borosilicate glass

Borosilicate glass Replace some of the Si sites with B

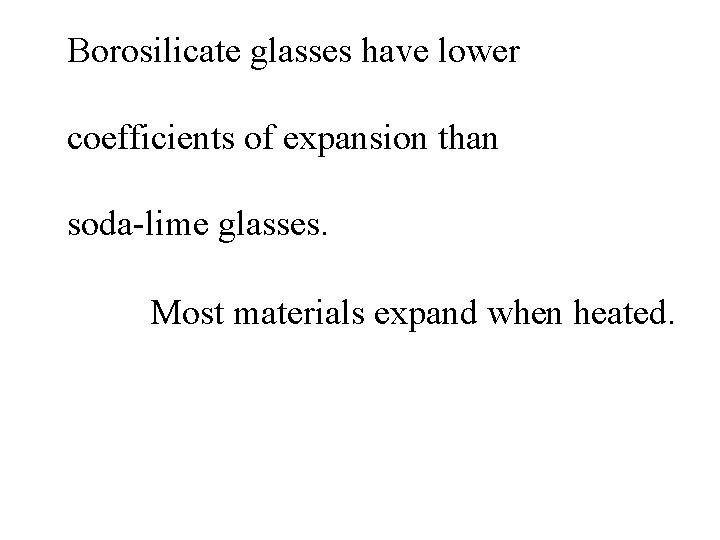
Borosilicate glasses have lower coefficients of expansion than soda-lime glasses.

Borosilicate glasses have lower coefficients of expansion than soda-lime glasses. Most materials expand when heated.

Most materials expand when heated. The coefficient of expansion is a factor, which when multiplied by the temperature change, gives the amount a material will expand or contract.

Since glasses are quite brittle, they are less likely to break when the temperature changes if they have a relatively low coefficient of expansion.

Borosilicate glasses have higher melting points than soda-lime glasses.

Borosilicate glasses have higher melting points than soda-lime glasses. Soda-lime glasses can be melted using a flame generated from methane and air.

Borosilicate glasses have higher melting points than soda-lime glasses. Soda-lime glasses can be melted using a flame generated from methane and air. It is necessary to use a methane/oxygen flame to work borosilicate glass.

Cements:

Cements: Portland cement is a specifically formulated powder.

Cements: Portland cement is a specifically formulated powder. When mixed with the proper amount of water it first forms a slurry which flows and can be formed.

When mixed with the proper amount of water it first forms a slurry which flows and can be formed. The slurry hardens and gains strength by the growth of a network of silicate crystals.
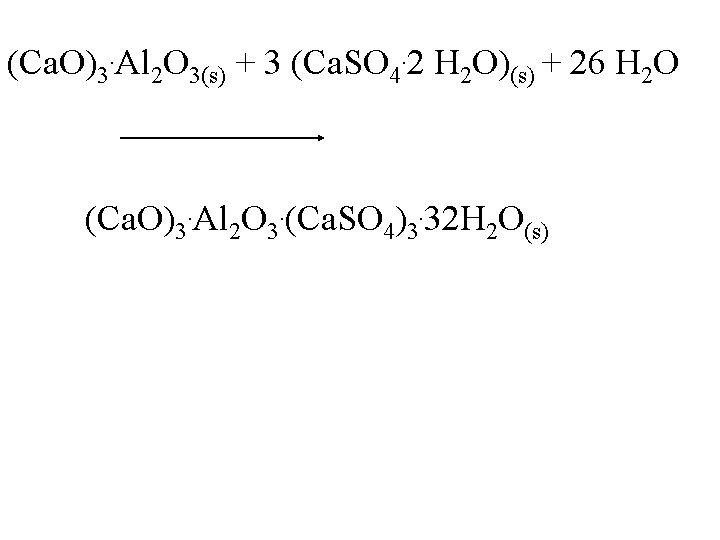
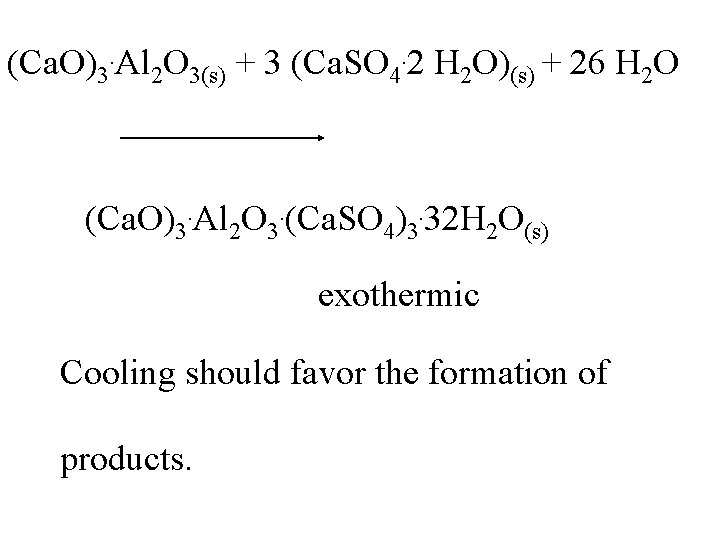
(Ca. O)3. Al 2 O 3(s) + 3 (Ca. SO 4. 2 H 2 O)(s) + 26 H 2 O (Ca. O)3. Al 2 O 3. (Ca. SO 4)3. 32 H 2 O(s)

(Ca. O)3. Al 2 O 3(s) + 3 (Ca. SO 4. 2 H 2 O)(s) + 26 H 2 O (Ca. O)3. Al 2 O 3. (Ca. SO 4)3. 32 H 2 O(s) exothermic

(Ca. O)3. Al 2 O 3(s) + 3 (Ca. SO 4. 2 H 2 O)(s) + 26 H 2 O (Ca. O)3. Al 2 O 3. (Ca. SO 4)3. 32 H 2 O(s) exothermic Cooling should favor the formation of products.
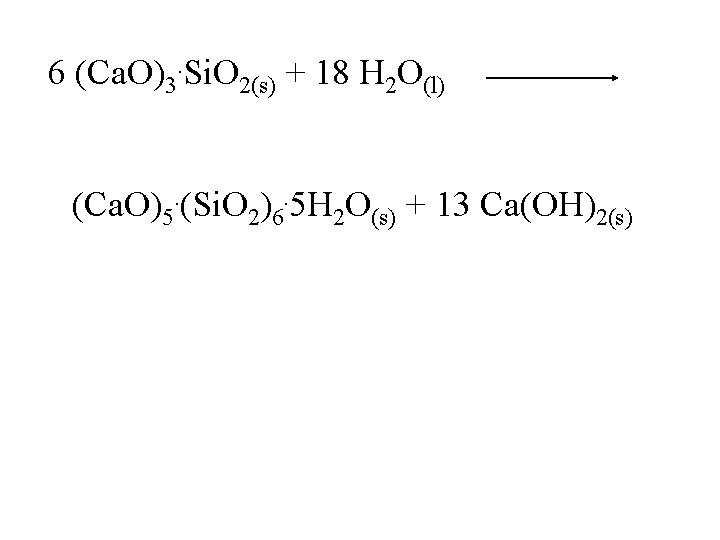
6 (Ca. O)3. Si. O 2(s) + 18 H 2 O(l) (Ca. O)5. (Si. O 2)6. 5 H 2 O(s) + 13 Ca(OH)2(s)
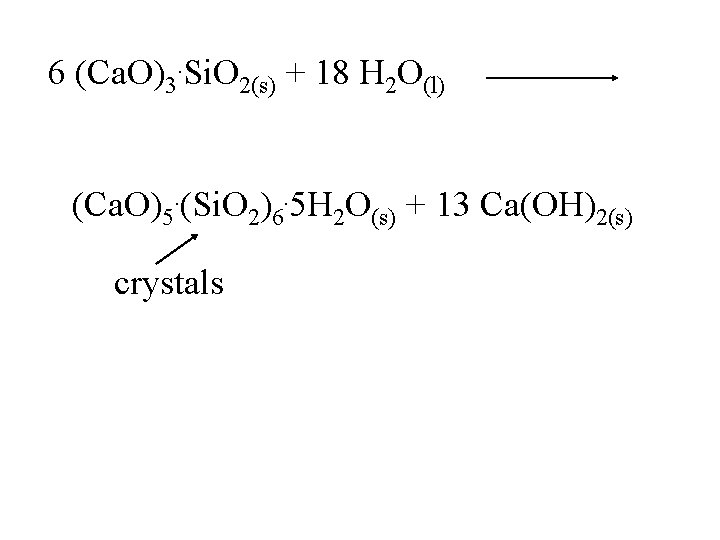
6 (Ca. O)3. Si. O 2(s) + 18 H 2 O(l) (Ca. O)5. (Si. O 2)6. 5 H 2 O(s) + 13 Ca(OH)2(s) crystals

6 (Ca. O)3. Si. O 2(s) + 18 H 2 O(l) (Ca. O)5. (Si. O 2)6. 5 H 2 O(s) + 13 Ca(OH)2(s) crystals If the mixture is allowed to dry too rapidly, sufficient water and time will not be available for crystal growth.
 Grade 7 natural science term 2 worksheets
Grade 7 natural science term 2 worksheets Periodic table metals and nonmetals
Periodic table metals and nonmetals Natural science grade 7 matter and materials
Natural science grade 7 matter and materials Ferrous metals vs non ferrous metals
Ferrous metals vs non ferrous metals Metalloids
Metalloids Metal and non metal
Metal and non metal Architecture frameworks
Architecture frameworks Liberta hax
Liberta hax Interpretive framework example
Interpretive framework example Food security concepts and frameworks
Food security concepts and frameworks What is a knowledge framework
What is a knowledge framework Nursing theories and conceptual frameworks
Nursing theories and conceptual frameworks Software architecture frameworks
Software architecture frameworks Enterprise agile frameworks
Enterprise agile frameworks Nursing informatics theories, models and frameworks
Nursing informatics theories, models and frameworks Php frameworks
Php frameworks Topic sentence
Topic sentence Describe trust frameworks.
Describe trust frameworks. Java e commerce frameworks
Java e commerce frameworks Interpretive framework
Interpretive framework Why i hate frameworks
Why i hate frameworks Net frameworks 4
Net frameworks 4 Non traditional art
Non traditional art Ethical and legal frameworks in nursing
Ethical and legal frameworks in nursing Actor frameworks
Actor frameworks List of theoretical frameworks
List of theoretical frameworks Regional construction frameworks
Regional construction frameworks Social studies frameworks
Social studies frameworks Local development frameworks
Local development frameworks Parcc model content frameworks
Parcc model content frameworks Conative messaging strategy
Conative messaging strategy Archaic pronouns
Archaic pronouns Self initiated other repair examples
Self initiated other repair examples Jordan yelinek
Jordan yelinek Metallic bonds
Metallic bonds Law of intermediate metals
Law of intermediate metals Memory metals
Memory metals Limitations of classical free electron theory
Limitations of classical free electron theory Sand mold casting
Sand mold casting Extrusion and drawing difference
Extrusion and drawing difference Chapter 7 ionic compounds and metals assessment answer key
Chapter 7 ionic compounds and metals assessment answer key Alkaline earth metals lewis dot structure
Alkaline earth metals lewis dot structure Gmaw electrode classification
Gmaw electrode classification What's morphology
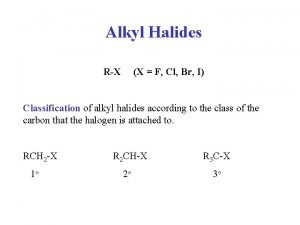
What's morphology R-x alkyl halide
R-x alkyl halide Poem about metals nonmetals and metalloids
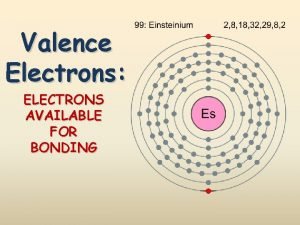
Poem about metals nonmetals and metalloids Valence electrons for transition metals
Valence electrons for transition metals Extraction of metals class 10
Extraction of metals class 10 Compare metals nonmetals and metalloids
Compare metals nonmetals and metalloids Alkali metals reacting with water
Alkali metals reacting with water Fun facts about alkali metals
Fun facts about alkali metals Viscietākais metāls ir
Viscietākais metāls ir Least reactive non-metal
Least reactive non-metal Elements and their properties chapter 17
Elements and their properties chapter 17 Metals tend to be
Metals tend to be What element burns with a squeaky pop
What element burns with a squeaky pop Magnesium with hot water
Magnesium with hot water Metals lose electrons
Metals lose electrons