Classifying the Elements Squares on the Periodic Table
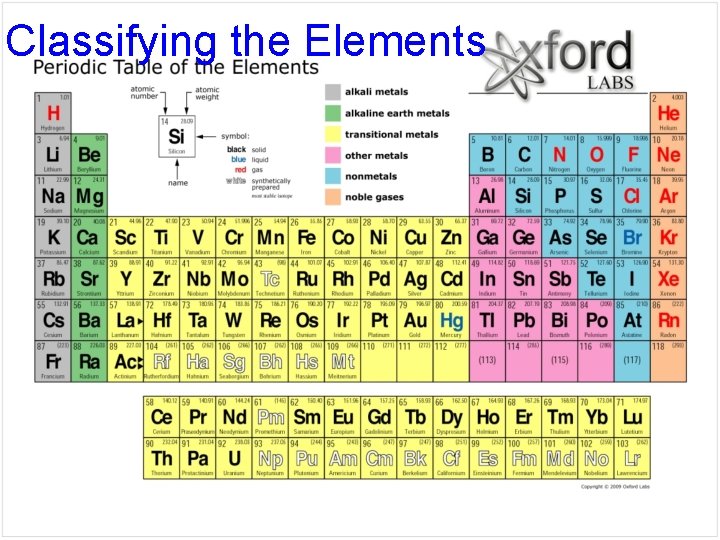
Classifying the Elements
Squares on the Periodic Table • The periodic table displays the chemical symbols of elements, along with information about the structure of their atom. Look at Carbon on the periodic table in your reference table. • What number is in bold in the lower left hand corner of that square? What can you tell me about this number? – 6. This is Carbon atomic number, or the number of protons contained in the nucleus of a carbon atom. • What is the number on the top left hand corner? What can you tell me about this number? – 12. 0111 This is carbons atomic mass, calculated by summing up the products of each carbon isotopes mass with their natural percent abundance • What are the numbers below the bolded number? What can you tell me about these numbers? – 2 -4 This is carbons electron configuration. It has 2 electrons located in both 1 s and 2 s orbitals and 2 in the 2 p sublevel.

Distinguishing Groups • Group 1 (1 A) – Alkali metals • Group 2 (2 A) – Alkaline Earth metals • Group 17 (7 A) – Halogens • Group 18 (8 A) - Noble Gases Elements can be sorted into noble gases, representative elements, transition metals or inner transition metals based on their electron configuration.

Noble Gases • Inert gases – They rarely take part in a reaction. • Their s and p sublevels are completely filled and due to this, they are stable Name a few noble gases: Helium, Neon, Argon, Krypton, Xenon, and Radon

Representative Elements • Elements in groups 1 -2 & 13 -18 (Group A elements) • Have a wide range of physical and chemical properties. • Include metals, metalloids and nonmetals. • The s and p sublevels are the highest occupied energy levels. • Can you a name a few representative elements? – Lithium, Chlorine, Nitrogen, etc…
Transition Metals and Inner Transition Metals • Transition Metals – Located in groups 3 -12 (B group elements) – Characterized by the presence of electrons in the d orbitals. Can you name a few transition metals? Iron, Cobalt, Nickel, etc. . • Inner Transition Metals – Located below the main body of the periodic table. – Characterized by the presence of electrons in the f orbitals. Since these elements have been omitted from physical properties page of your reference tables, we won’t focus on these elements.
Where are the sublevels located on the periodic table. • In which groups are the s-block elements found? 1 & 2 (1 A & 2 A) • In which groups are the p-block elements found? 13 -18 (3 A – 8 A) • In which groups are the d-block elements found? 3 -12 (Group B Elements) • Where are the f-block elements found? Below the periodic table.

6. 2 pg. 173 Questions 14, 16, 17 Read 6. 3 pgs. 174 -181
- Slides: 8