CLASSIFYING REACTIONS Synthesis Reactions Here is another example



- Slides: 20

CLASSIFYING REACTIONS

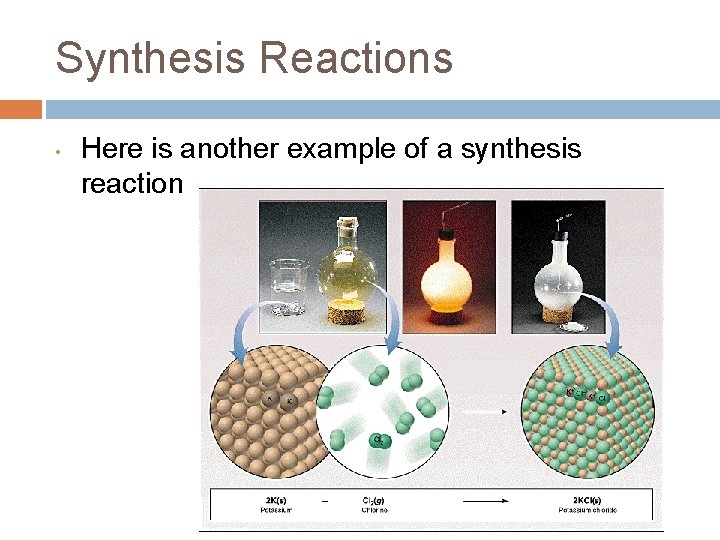
Synthesis Reactions • Here is another example of a synthesis reaction

1. Synthesis reactions • • Synthesis reactions occur when two substances (generally elements) combine and form a compound. (Sometimes these are called combination or addition reactions. ) reactant + reactant 1 product Basically: A + B AB • • Example: 2 H 2 + O 2 2 H 2 O Example: C + O 2 CO 2

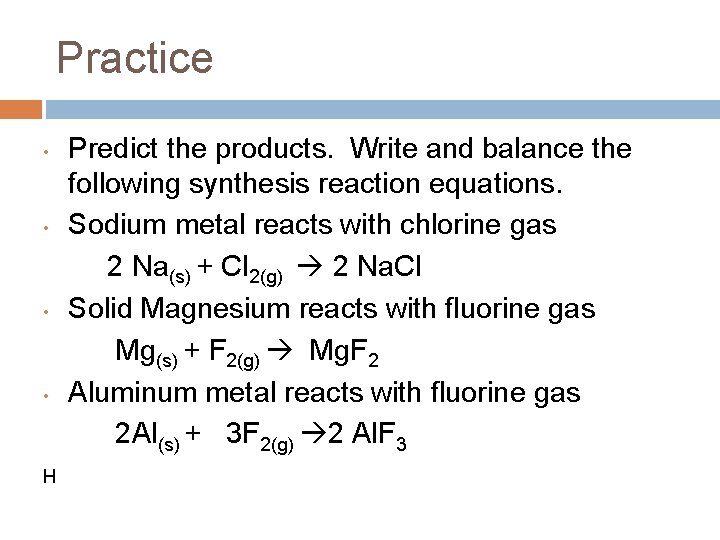
Practice • Predict the products. Write and balance the following synthesis reaction equations. Sodium metal reacts with chlorine gas • Solid Magnesium reacts with fluorine gas • Aluminum metal reacts with fluorine gas •

Practice • • H Predict the products. Write and balance the following synthesis reaction equations. Sodium metal reacts with chlorine gas 2 Na(s) + Cl 2(g) 2 Na. Cl Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g) Mg. F 2 Aluminum metal reacts with fluorine gas 2 Al(s) + 3 F 2(g) 2 Al. F 3

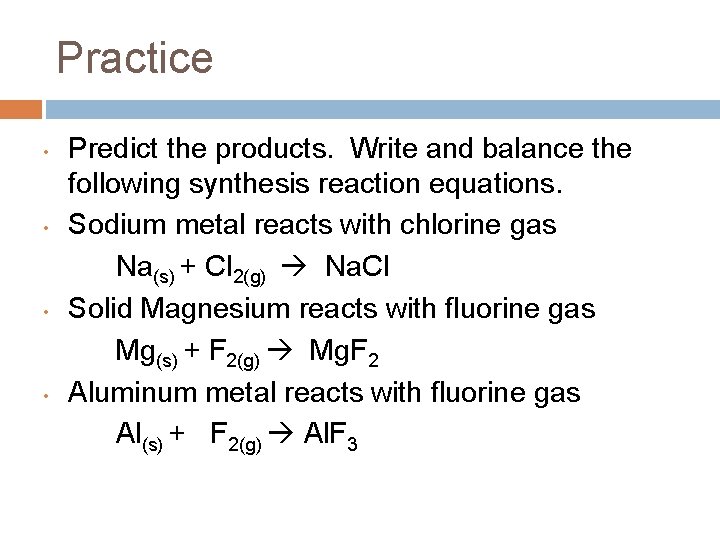
Practice • • Predict the products. Write and balance the following synthesis reaction equations. Sodium metal reacts with chlorine gas Na(s) + Cl 2(g) Na. Cl Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g) Mg. F 2 Aluminum metal reacts with fluorine gas Al(s) + F 2(g) Al. F 3

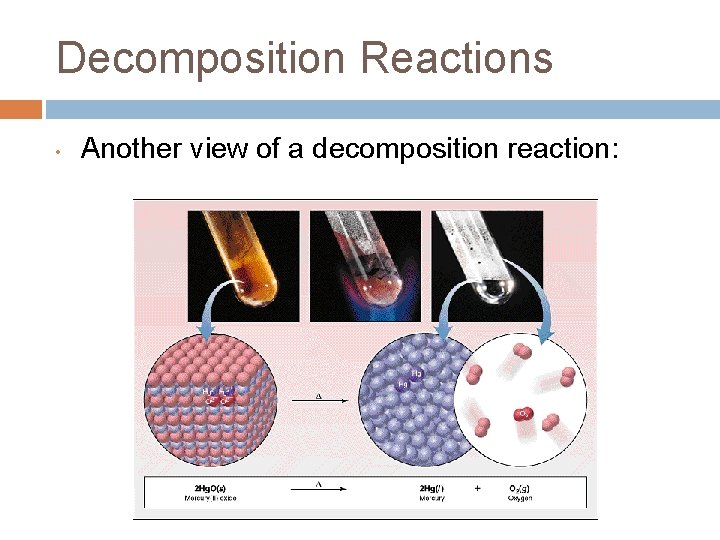
Decomposition Reactions • Another view of a decomposition reaction:

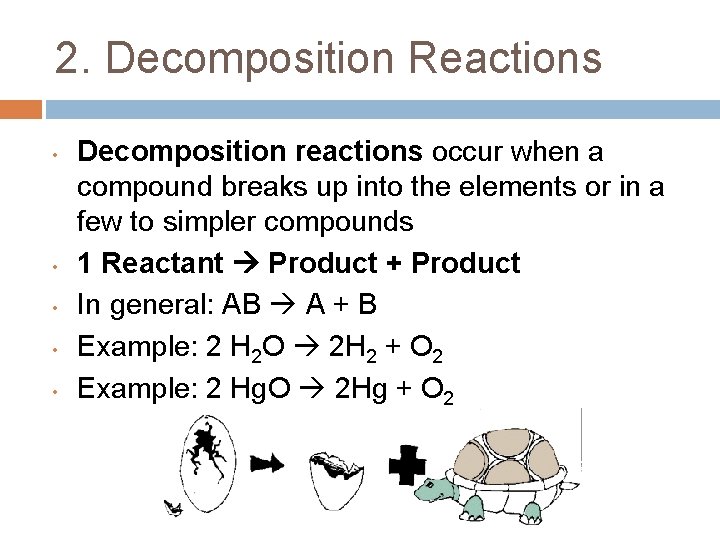
2. Decomposition Reactions • • • Decomposition reactions occur when a compound breaks up into the elements or in a few to simpler compounds 1 Reactant Product + Product In general: AB A + B Example: 2 H 2 O 2 H 2 + O 2 Example: 2 Hg. O 2 Hg + O 2

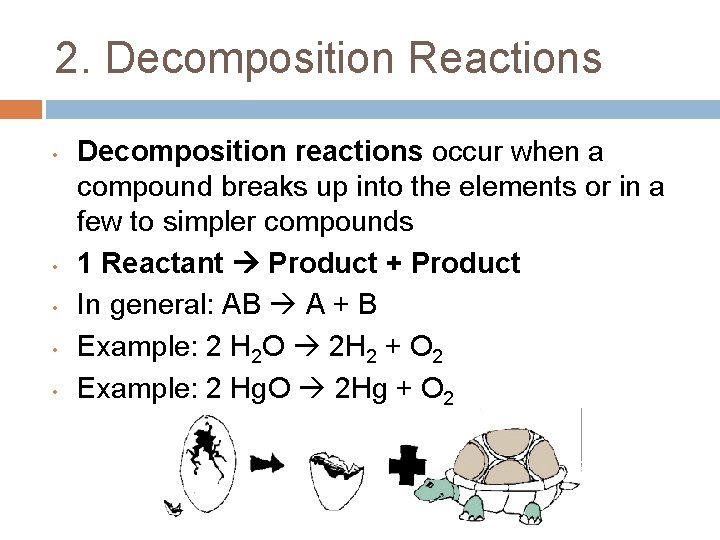
2. Decomposition Reactions • • • Decomposition reactions occur when a compound breaks up into the elements or in a few to simpler compounds 1 Reactant Product + Product In general: AB A + B Example: 2 H 2 O 2 H 2 + O 2 Example: 2 Hg. O 2 Hg + O 2

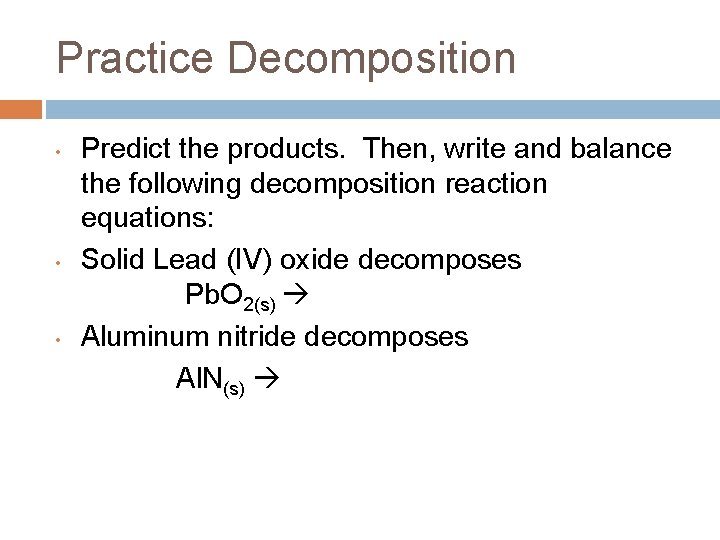
Practice Decomposition • • • Predict the products. Then, write and balance the following decomposition reaction equations: Solid Lead (IV) oxide decomposes Pb. O 2(s) Aluminum nitride decomposes Al. N(s)

Practice • • • Predict the products. Then, write and balance the following decomposition reaction equations: Solid Lead (IV) oxide decomposes Pb. O 2(s) Pb + O 2 Aluminum nitride decomposes 2 Al. N(s) 2 Al + N 2

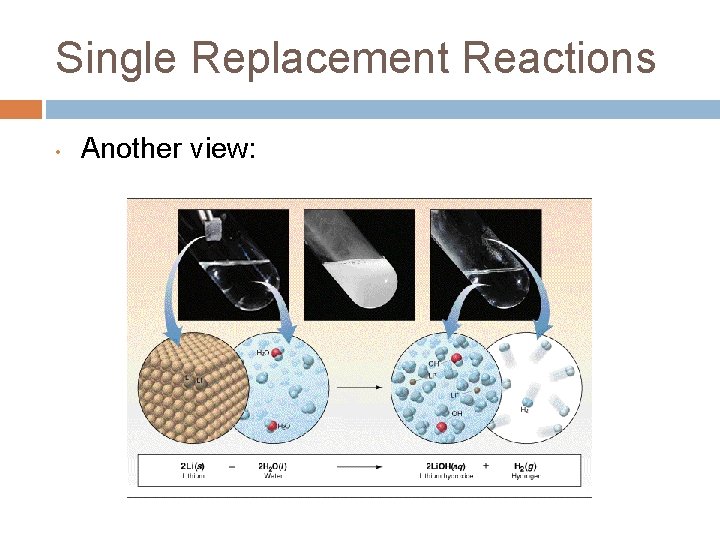
Single Replacement Reactions • Another view:

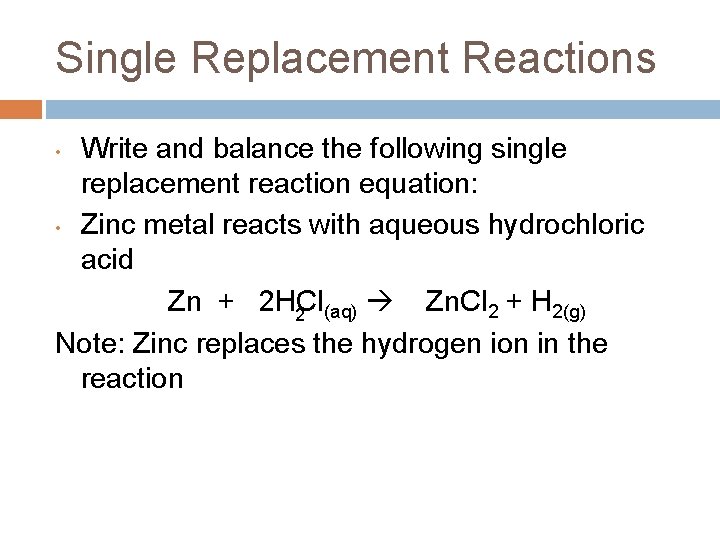
Single Replacement Reactions • • Write and balance the following single replacement reaction equation: Zinc metal reacts with aqueous hydrochloric acid 2 Note: Zinc replaces the hydrogen ion in the reaction

Single Replacement Reactions Write and balance the following single replacement reaction equation: • Zinc metal reacts with aqueous hydrochloric acid Zn + 2 HCl Zn. Cl 2 + H 2(g) 2 (aq) Note: Zinc replaces the hydrogen ion in the reaction •

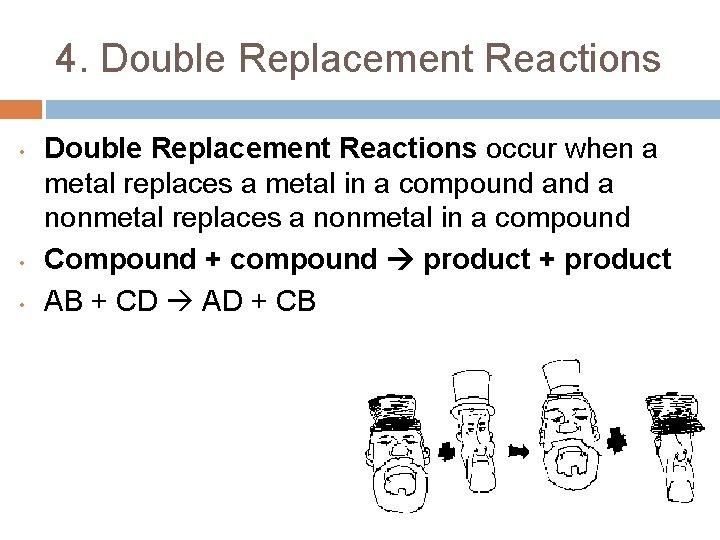
4. Double Replacement Reactions • • • Double Replacement Reactions occur when a metal replaces a metal in a compound a nonmetal replaces a nonmetal in a compound Compound + compound product + product AB + CD AD + CB

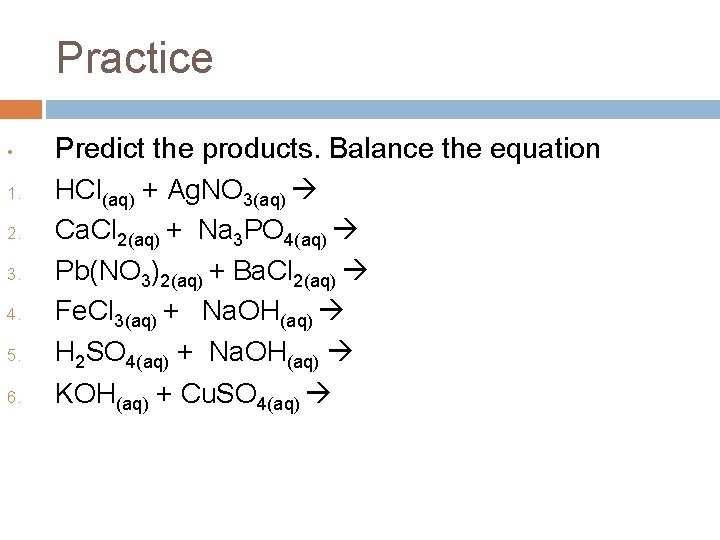
Practice • 1. 2. 3. 4. 5. 6. Predict the products. Balance the equation HCl(aq) + Ag. NO 3(aq) Ca. Cl 2(aq) + Na 3 PO 4(aq) Pb(NO 3)2(aq) + Ba. Cl 2(aq) Fe. Cl 3(aq) + Na. OH(aq) H 2 SO 4(aq) + Na. OH(aq) KOH(aq) + Cu. SO 4(aq)

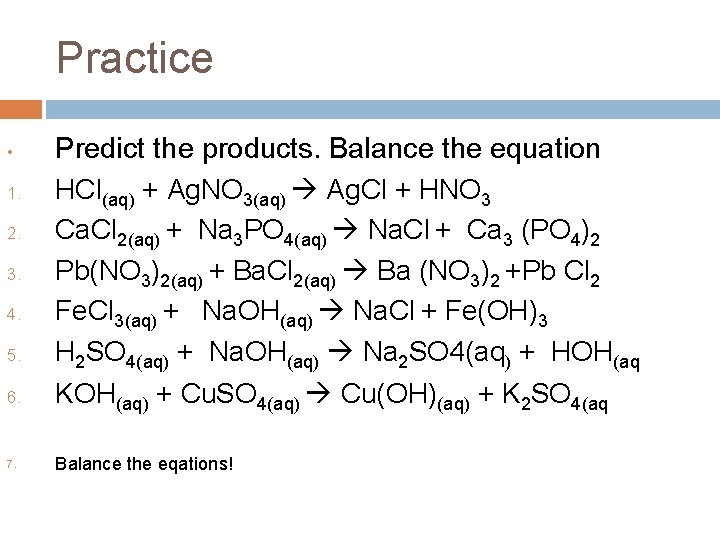
Practice • 1. 2. 3. 4. 5. 6. 7. Predict the products. Balance the equation HCl(aq) + Ag. NO 3(aq) Ag. Cl + HNO 3 Ca. Cl 2(aq) + Na 3 PO 4(aq) Na. Cl + Ca 3 (PO 4)2 Pb(NO 3)2(aq) + Ba. Cl 2(aq) Ba (NO 3)2 +Pb Cl 2 Fe. Cl 3(aq) + Na. OH(aq) Na. Cl + Fe(OH)3 H 2 SO 4(aq) + Na. OH(aq) Na 2 SO 4(aq) + HOH(aq KOH(aq) + Cu. SO 4(aq) Cu(OH)(aq) + K 2 SO 4(aq Balance the eqations!

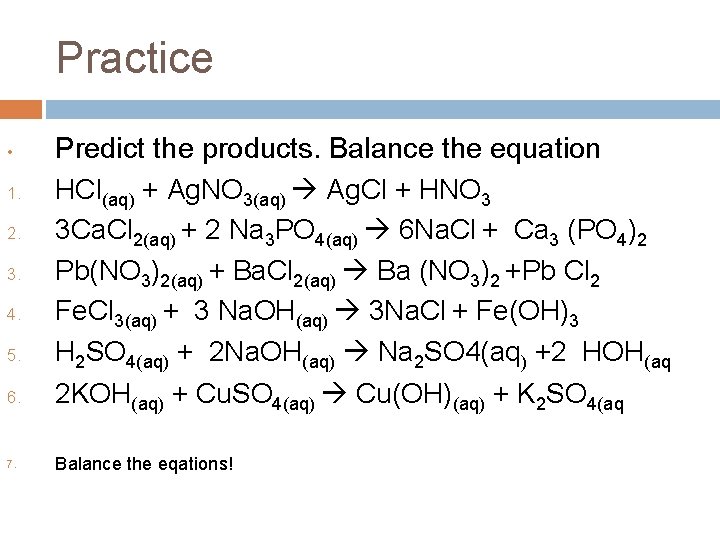
Practice • 1. 2. 3. 4. 5. 6. 7. Predict the products. Balance the equation HCl(aq) + Ag. NO 3(aq) Ag. Cl + HNO 3 3 Ca. Cl 2(aq) + 2 Na 3 PO 4(aq) 6 Na. Cl + Ca 3 (PO 4)2 Pb(NO 3)2(aq) + Ba. Cl 2(aq) Ba (NO 3)2 +Pb Cl 2 Fe. Cl 3(aq) + 3 Na. OH(aq) 3 Na. Cl + Fe(OH)3 H 2 SO 4(aq) + 2 Na. OH(aq) Na 2 SO 4(aq) +2 HOH(aq 2 KOH(aq) + Cu. SO 4(aq) Cu(OH)(aq) + K 2 SO 4(aq Balance the eqations!

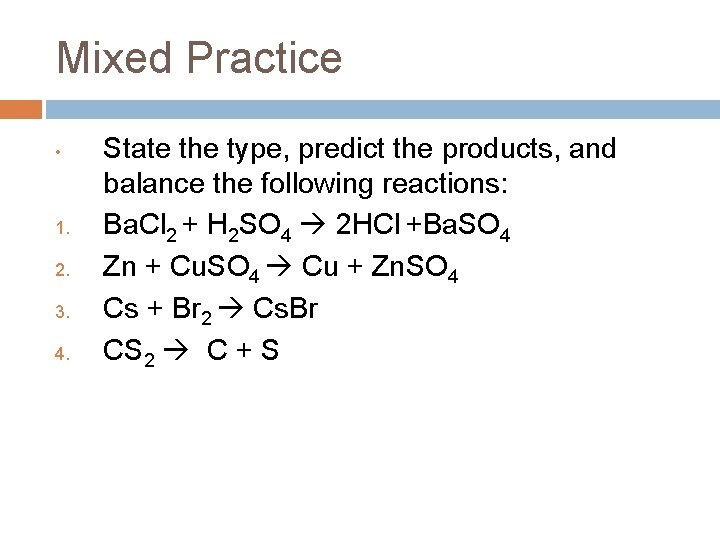
Mixed Practice • 1. 2. 3. 4. State the type, predict the products, and balance the following reactions: Ba. Cl 2 + H 2 SO 4 Zn + Cu. SO 4 Cs + Br 2 Fe. CO 3

Mixed Practice • 1. 2. 3. 4. State the type, predict the products, and balance the following reactions: Ba. Cl 2 + H 2 SO 4 2 HCl +Ba. SO 4 Zn + Cu. SO 4 Cu + Zn. SO 4 Cs + Br 2 Cs. Br CS 2 C + S