Classifying Matter The final Stage Matter Pure Substances

Classifying Matter The final Stage
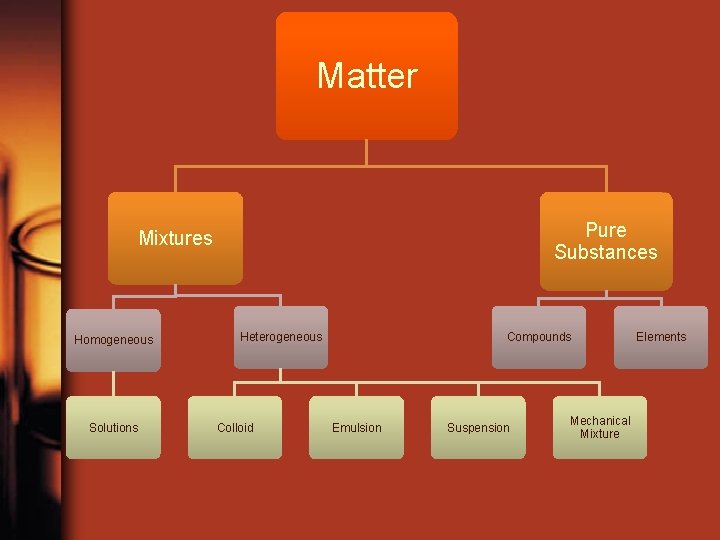
Matter Pure Substances Mixtures Homogeneous Solutions Heterogeneous Colloid Compounds Emulsion Suspension Mechanical Mixture Elements
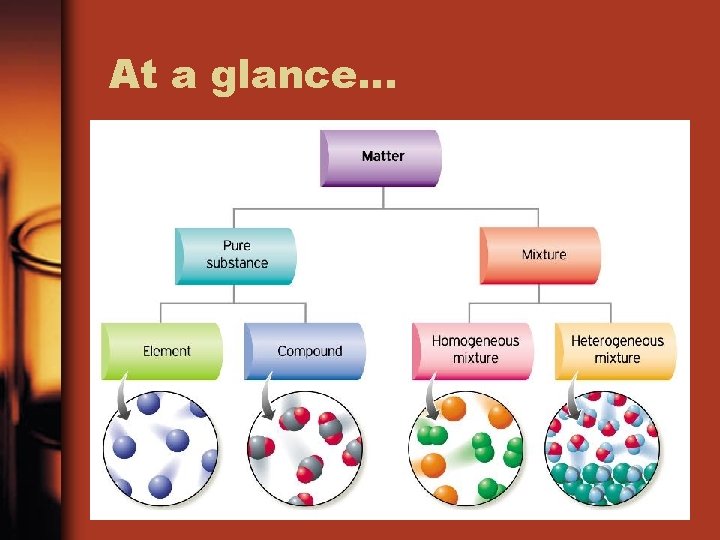
At a glance…

Pure Substance or Mixture? • Pure Substance: a substance that contains only one kind of matter; and has a fixed composition • Mixture: substance consisting of two or more substances mixed together

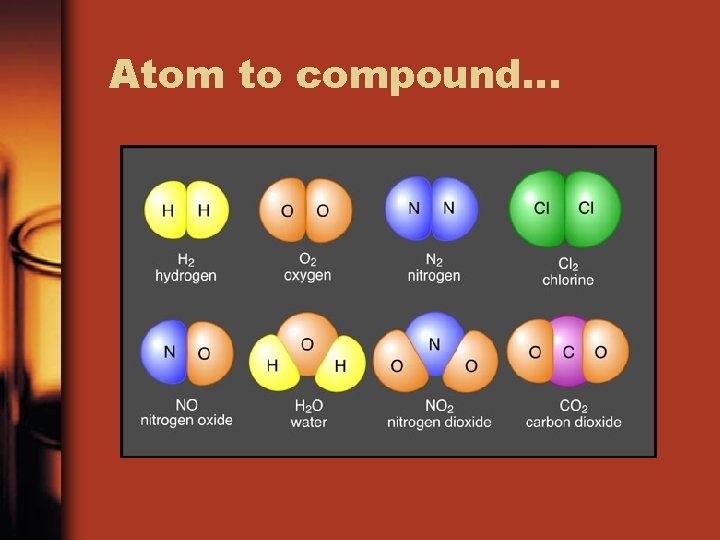
Pure substances: Element or Compound • Element: From the Periodic table, substances that are made of 1 type of atom and cannot be separated into simpler substances. – Includes diatomic elements. Diatomic means two exact elements together like O 2 • Compound: more than one non diatomic element chemically combined in a fixed composition
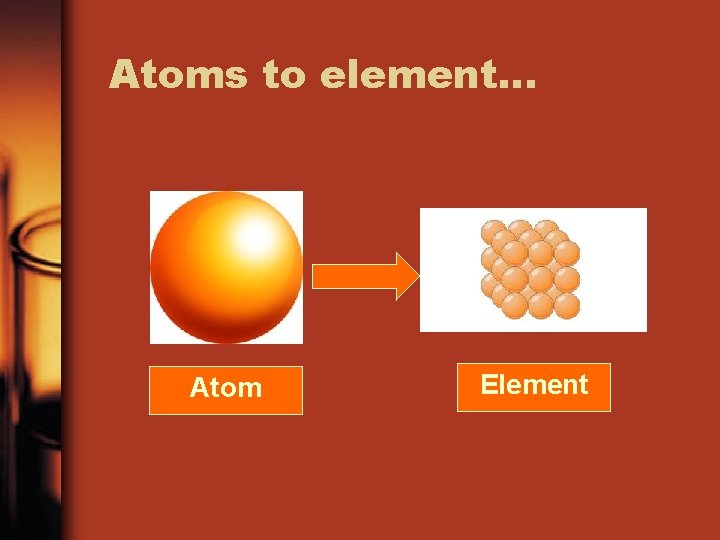
Atoms to element… Atom Element

Element n o rb a C Sulp hur
Atom to compound…

Compound: fixed composition

A fixed composition? • Law of definite proportions: Elements and compounds are made up of 2 or more elements combined together in definite ratios(proportions) • Table salt will always contain 1 sodium atom to 1 chlorine atom • Na. Cl has 1 Na : 1 Cl • H 2 O 2 has 2 H : 2 O
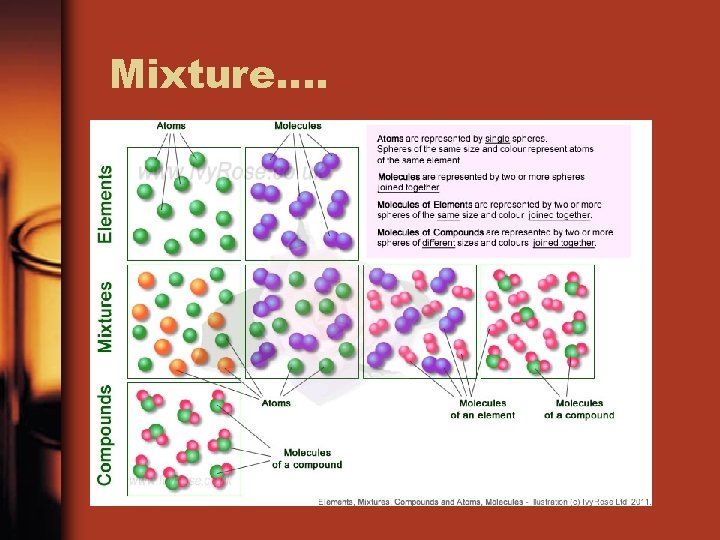
Mixture….

Mixture: Heterogeneous or Homogeneous • “Heterogeneous mixture” means seeing different kinds • “Homogeneous mixture”: seeing one kind (solution)

Homogeneous Mixture • Mix of two or more pure substances that mix together so well it looks like only one substance • 1 Phase • Example: kool-aid or the alloy steel

Heterogeneous Mixture • A mixture containing two or more substances that are visible/separate • Examples: Density column, soil, pile of dice

Types: • Ordinary mechanical mixture – Can identify various visible solid components. – Easily separated • Examples: pizza, salad, soil.

Heterogeneous Mixtures • Suspension: a heterogeneous fluid containing solid particles that are sufficiently large for sedimentation. (Ex. Flour in water) • Colloid: a substance microscopically dispersed evenly throughout another one (Ex. Milk) • Emulsion: a mixture of two or more immiscible (unblendable) liquids (Ex. Vinagrette
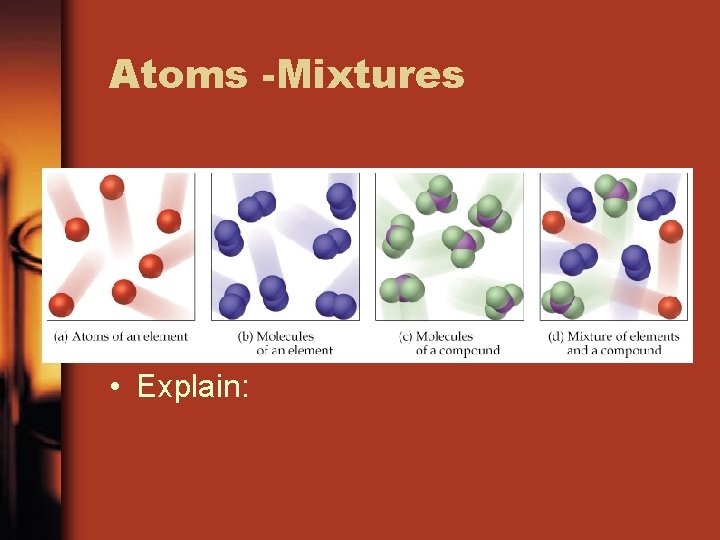
Atoms -Mixtures • Explain:
- Slides: 17