Classifying Matter Science 7 Chapter 10 Lesson 1



- Slides: 13

Classifying Matter Science 7— Chapter 10— Lesson 1 Lecture Not es May 12, 201 5

Understanding Matter 0 Matter is anything that has mass and takes up space 0 Everything is matter. {Exceptions—light, magnetism} 0 An atom is a small particle that is a building block of matter

Atoms 0 Atoms have 3 parts • protons • neutrons • electrons o At the center of an atom is a nucleus. The nucleus has a positive charge. o Protons have a positive charge and are found in the nucleus.

Atoms o Neutrons have no charge (neutral) charge and are found in the nucleus. o Electrons have a negative charge and are found in the electron cloud o An atom is almost too small to imagine.

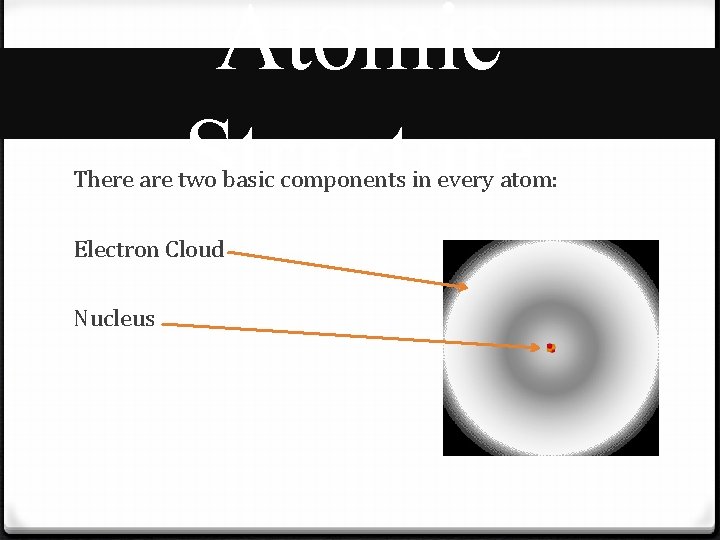
Atomic Structure There are two basic components in every atom: Electron Cloud Nucleus

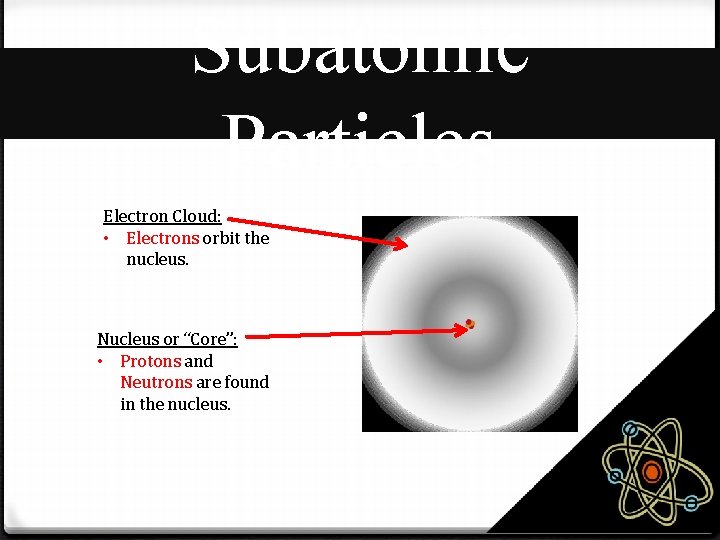
Subatomic Particles Electron Cloud: • Electrons orbit the nucleus. Nucleus or “Core”: • Protons and Neutrons are found in the nucleus.

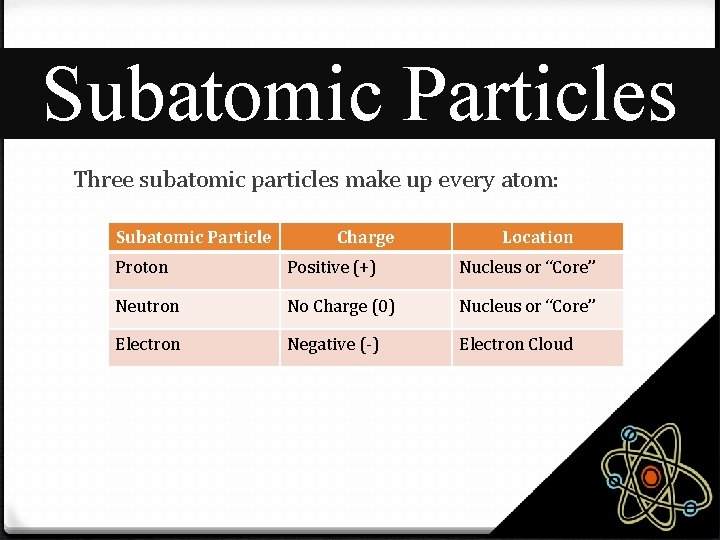
Subatomic Particles Three subatomic particles make up every atom: Subatomic Particle Charge Location Proton Positive (+) Nucleus or “Core” Neutron No Charge (0) Nucleus or “Core” Electron Negative (-) Electron Cloud

Atoms 0 Matter is anything that takes up space and has mass. All matter is made of atoms. 0 Atoms are the basic building blocks of matter. They make up everything around us; Your desk, the board, your body, everything is made of atoms! 0 Atoms are too small to see without powerful microscopes.

Substances o. A substance is matter with a composition that is always the same. o. This means that a given substance is always made of the same combination(s) of atoms.

Elements o. An element is a substance that consists of just one type of atom oatomic number--the number of protons in an atom oatomic mass--the number of protons and neutrons in an atom (Round atomic mass to nearest one. )

Elements o. Protons = Atomic Number o. For an atom to be neutral, number of protons = number of electrons o. Neutrons = Atomic Mass (Rounded to nearest 1) – Atomic Number o. Symbol stands for the element. One capital letter or one capital letter and lower case letter.

Elements o. The most basic unit of matter is the element!

http: //www. brainpop. com/science/matterandchemistry/atom icmodel/