Classifying Matter Physical and Chemical Properties By Liz

Classifying Matter: Physical and Chemical Properties By Liz La. Rosa 5 th Grade Science www. middleschoolscience. com 2009
Matter Anything that has mass and volume Mass- amount of matter an object contains Measured in grams Volume- amount of space object occupies Measured in m. L or cm 3

Matter Sort the items on your table into groups. Make sure you sort using only one trait at a time. How did you sort? These “traits” or “characteristics” you used to sort the matter are called properties of matter. Some are more helpful then others.

Properties of Matter Physical Properties Any property that can be observed without changing the object/substance Anything that can be measured Which traits did you use to classify by that were physical? More Examples: Mass, volume, density Color, texture, shape Boiling point, freezing point
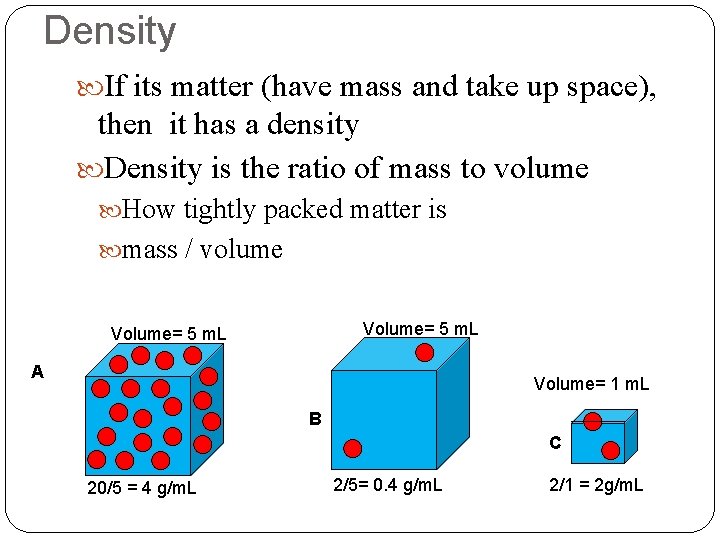
Density If its matter (have mass and take up space), then it has a density Density is the ratio of mass to volume How tightly packed matter is mass / volume Volume= 5 m. L A Volume= 1 m. L B C 20/5 = 4 g/m. L 2/5= 0. 4 g/m. L 2/1 = 2 g/m. L

Properties of Matter Chemical Properties A property of matter that can only be observed by altering the composition of the substance Have to “test” for it and risk changing the objects identitiy Which traits did you use to classify by that were physical? Examples: Flammability Reactivity
Flammability A material’s ability to burn in the presence of oxygen Chemical property Can only test for it Testing risks changing its identity and chemical makeup

Reactivity Describes a substance’s ability to participate in chemical reactions How readily (easily) a substance combines chemically with other substances. Chemical property Can only test for it Testing risks changing its identity and chemical makeup Can means many things Ex: React with acids React with lithium React with water Etc.
Characteristic Properties Aid most in identifying and classifying the substance Can be physical or chemical Are always there no matter the size or condition of the sample Examples of characteristic properties: Density Solubility (ability to dissolve in certain liquids) Reactivity flammability
Demonstration There are 3 clear liquids: Methanol Acetic Acid Water What properties do they have that are NOT helpful in identifying each? Color Liquid at room temperature Volume Viscosity What properties were most helpful? Flammability (Methanol) Reacts with Sodium Bicarbonate (Acetic Acid)
- Slides: 10