Classifying Matter Elements Compounds and Mixtures Each Starburst

Classifying Matter: Elements, Compounds, and Mixtures

Each Starburst represents an atom. What is an atom?

Atoms • Atom: the smallest particle that has the properties of an element.

Organize your Starbursts into piles by color.

Each pile of Starbursts represents an element. What is an element?
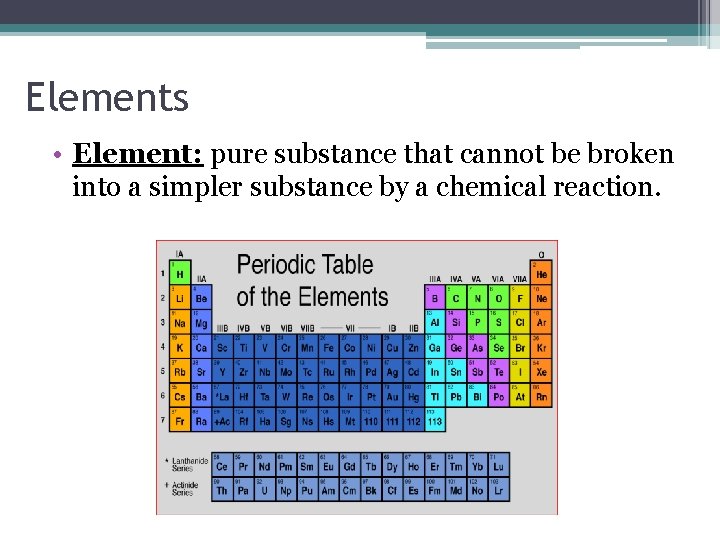
Elements • Element: pure substance that cannot be broken into a simpler substance by a chemical reaction.
What is a molecule? • Molecule: a pure substance composed of two or more atoms bonded together. ▫ Can be the same elements or different elements. ▫ Examples: H 2, O 2, N 2, H 2 O

Using your Starbursts and toothpicks, build 2 molecules. • One molecule should be made up of the same elements. • One molecule should be made up of different elements.
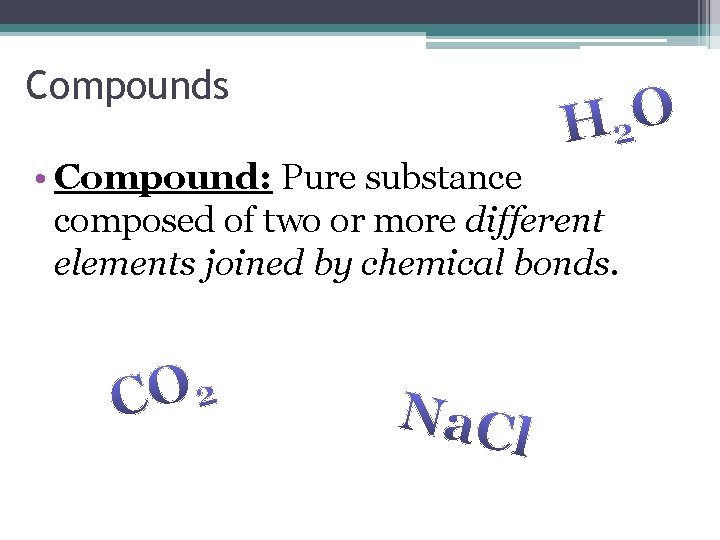
Compounds • Compound: Pure substance composed of two or more different elements joined by chemical bonds.

Pure Substances • Only one substance is present.

Use your Starbursts and toothpicks to create a mixture.
Mixtures • Mixture: A combination of two or more pure substances that are not chemically combined. Chem 4 kids. com

So, How Do You Tell The Difference Between Elements, Compounds, and Mixtures?
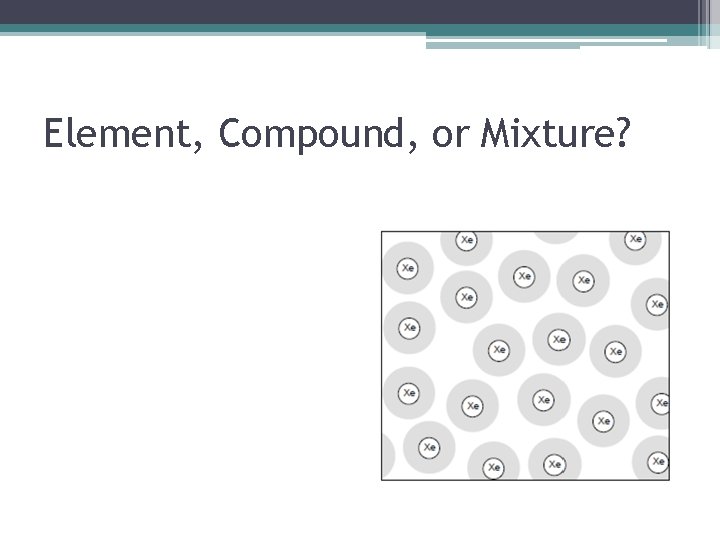
Element, Compound, or Mixture?
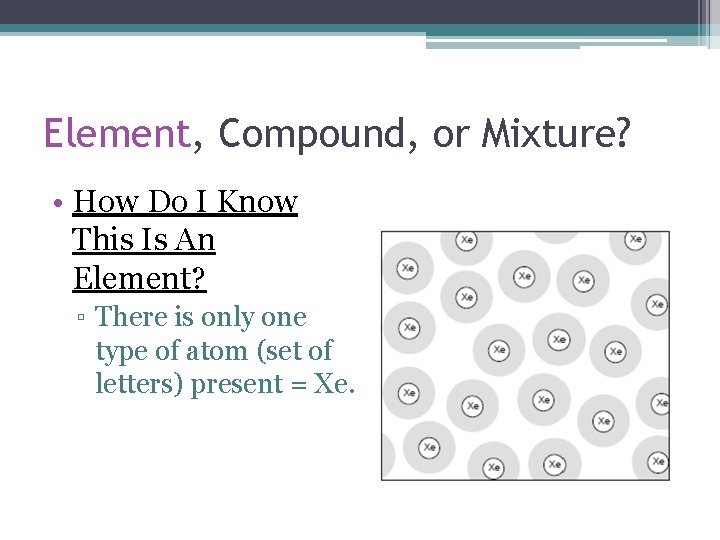
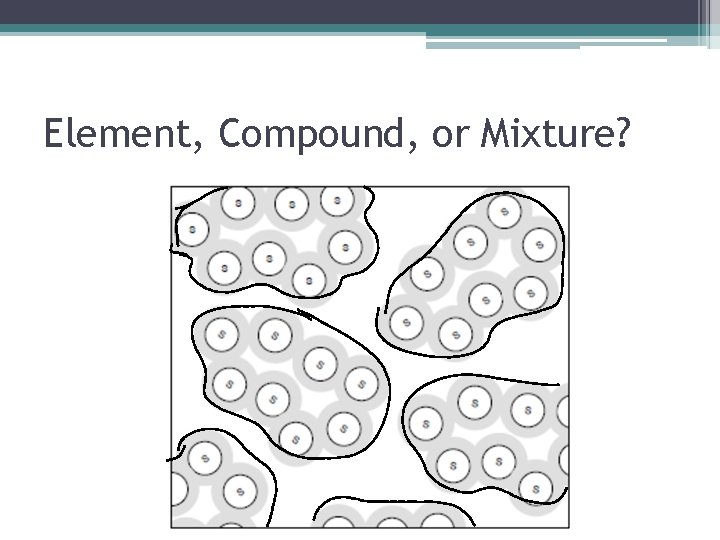
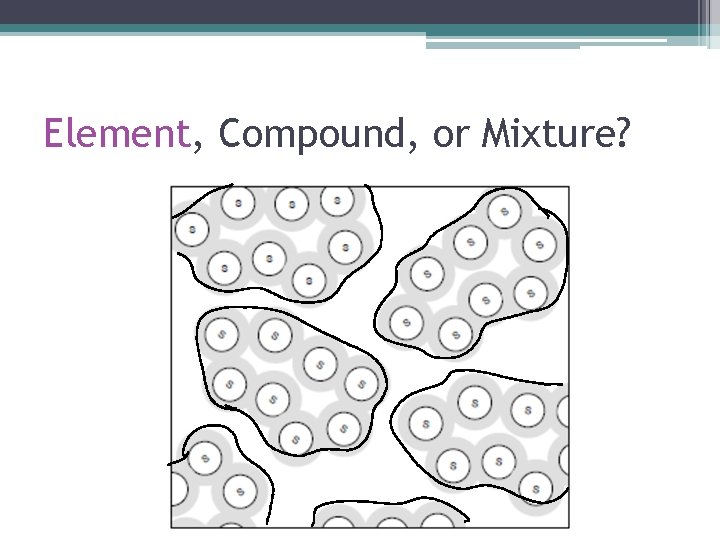
Element, Compound, or Mixture? • How Do I Know This Is An Element? ▫ There is only one type of atom (set of letters) present = Xe.

Element, Compound, or Mixture?
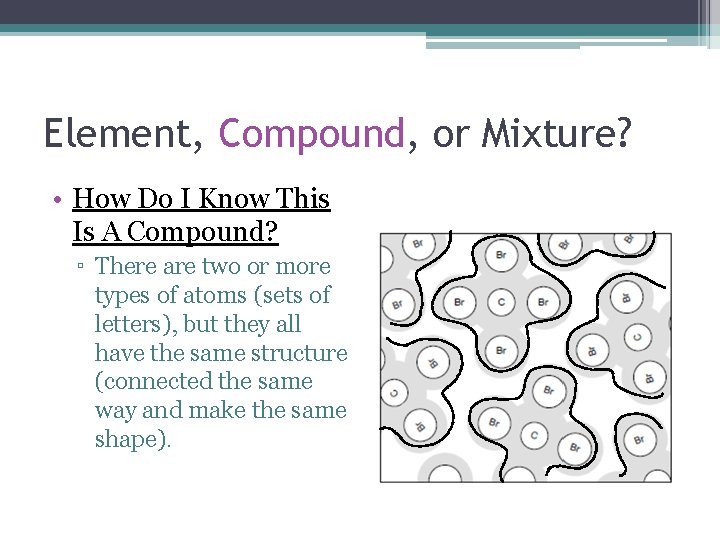
Element, Compound, or Mixture? • How Do I Know This Is A Compound? ▫ There are two or more types of atoms (sets of letters), but they all have the same structure (connected the same way and make the same shape).
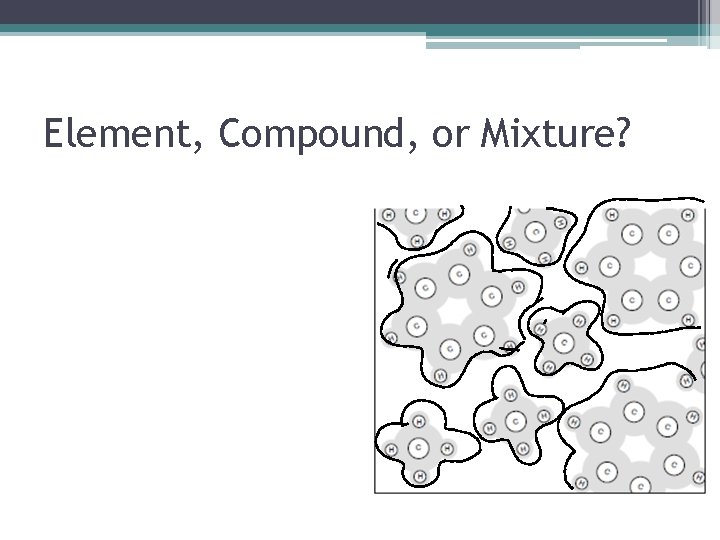
Element, Compound, or Mixture?
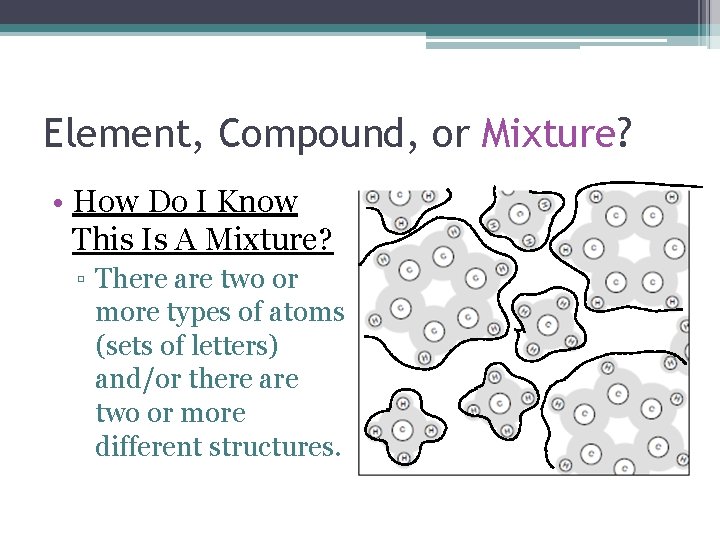
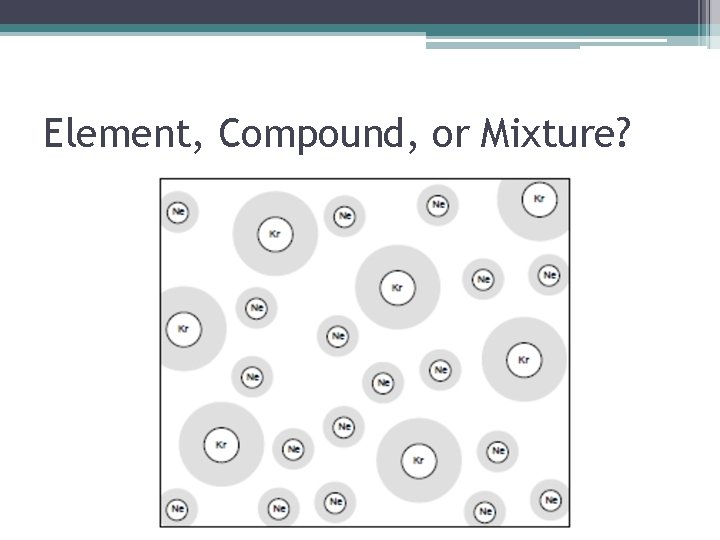
Element, Compound, or Mixture? • How Do I Know This Is A Mixture? ▫ There are two or more types of atoms (sets of letters) and/or there are two or more different structures.
Element, Compound, or Mixture?
Element, Compound, or Mixture?
Element, Compound, or Mixture?
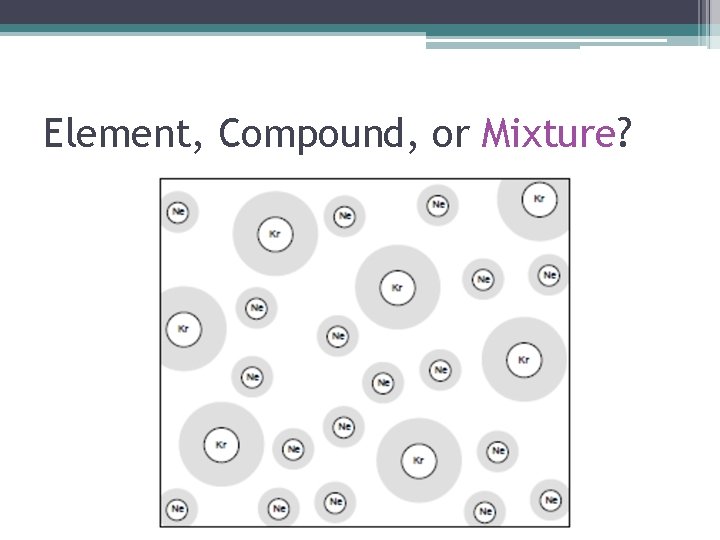
Element, Compound, or Mixture?
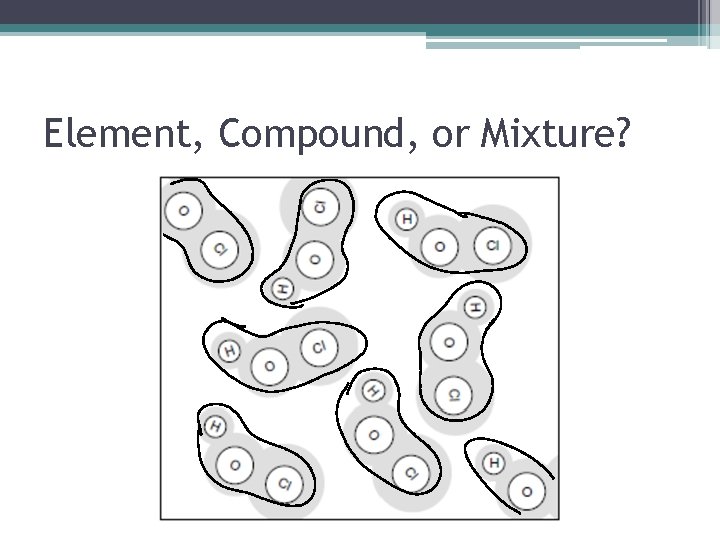
Element, Compound, or Mixture?

Element, Compound, or Mixture?
- Slides: 25