Classifying Matter Elements Compounds and Mixtures 8 th



































- Slides: 44

Classifying Matter: Elements, Compounds, and Mixtures 8 th Grade Science www. middleschoolscience. com 2009

What is matter? Matter is…. Anything that takes up space (volume) and has mass. Water Rocks Air Wood Plastic You

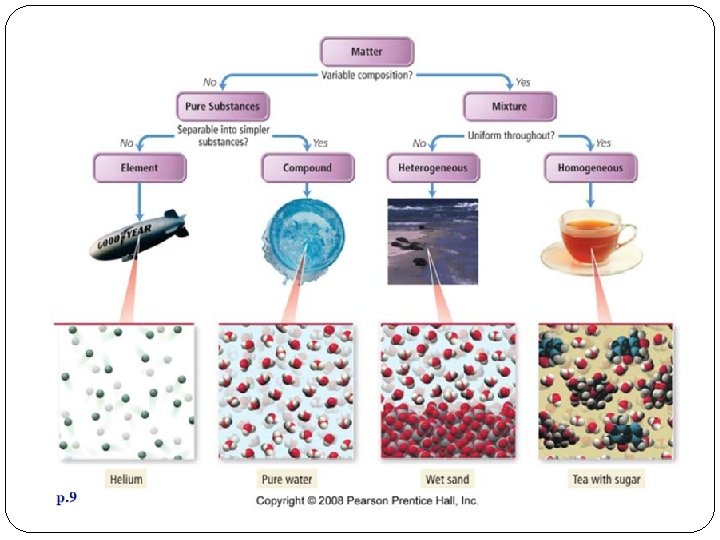
How is matter classified? Scientists like to classify things. One way that scientists classify matter is by its composition. Ultimately, all matter can be classified as elements, compounds and mixtures Elements and compounds are pure substances Mixtures are not pure substance

What are Pure Substances? A sample of matter that has a definite composition and definite chemical and physical properties

What are Elements? Made of one type of atom Cannot be separated into simpler substance by physical or chemical means. Located on the periodic table

What are atoms? The smallest part of an element Atoms are the basic building blocks of all matter Atoms chemically joined together are called molecules

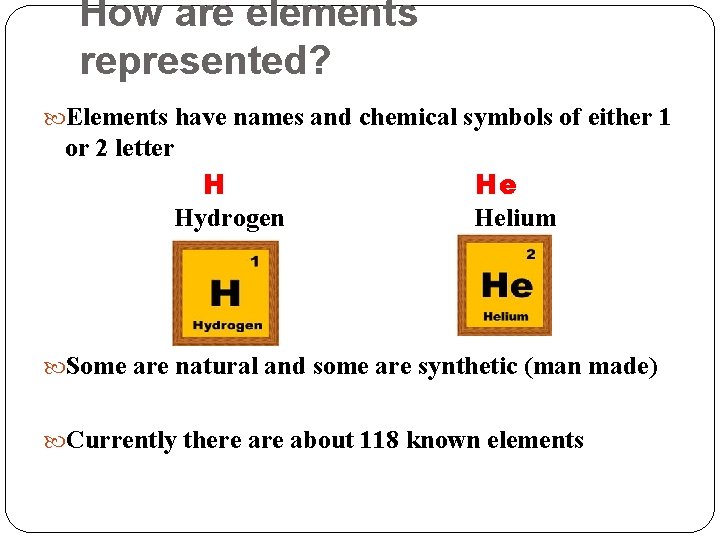
How are elements represented? Elements have names and chemical symbols of either 1 or 2 letter H Hydrogen He Helium Some are natural and some are synthetic (man made) Currently there about 118 known elements

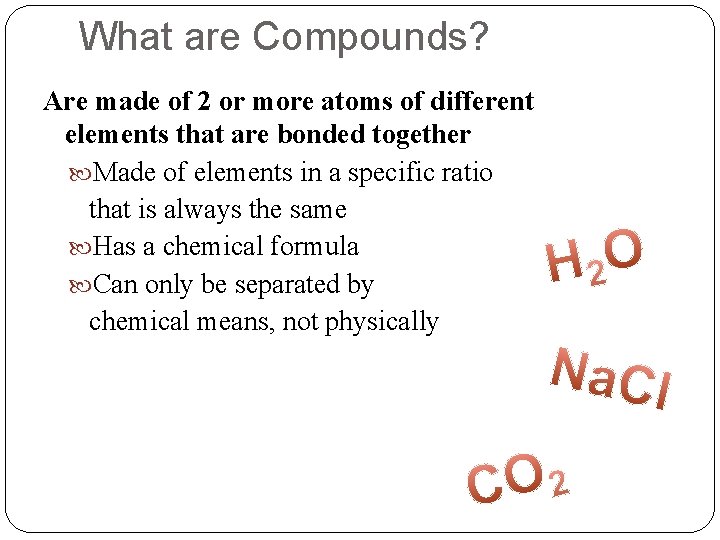
What are Compounds? Are made of 2 or more atoms of different elements that are bonded together Made of elements in a specific ratio that is always the same Has a chemical formula Can only be separated by chemical means, not physically

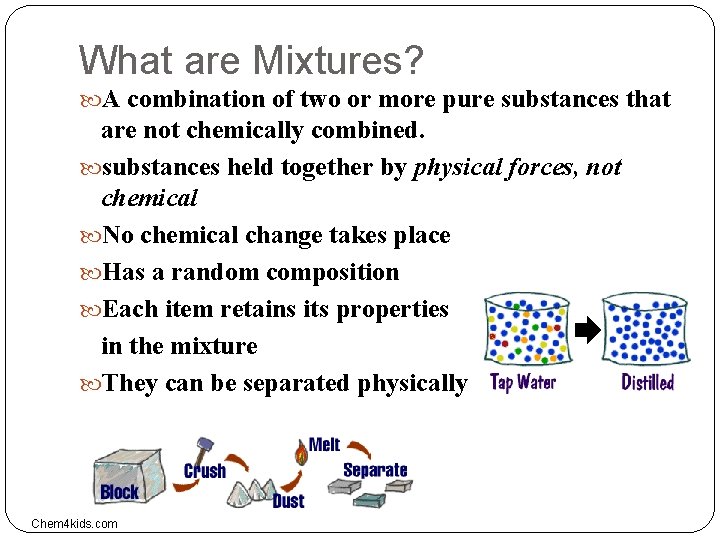
What are Mixtures? A combination of two or more pure substances that are not chemically combined. substances held together by physical forces, not chemical No chemical change takes place Has a random composition Each item retains its properties in the mixture They can be separated physically Chem 4 kids. com

Is it uniform throughout? Matter is also classified by its uniformity If the answer is no, the matter is a heterogeneous mixture. Considered the “least mixed. ” Does not appear to be the same throughout. Particles are large enough to be seen and to be separated from the mixture.

Is it uniform throughout? If the answer is yes, the matter is homogeneous (looks the same throughout). If it can’t be physically separated it’s a homogeneous mixture If it can not be separated then it is a pure substance –element or compound


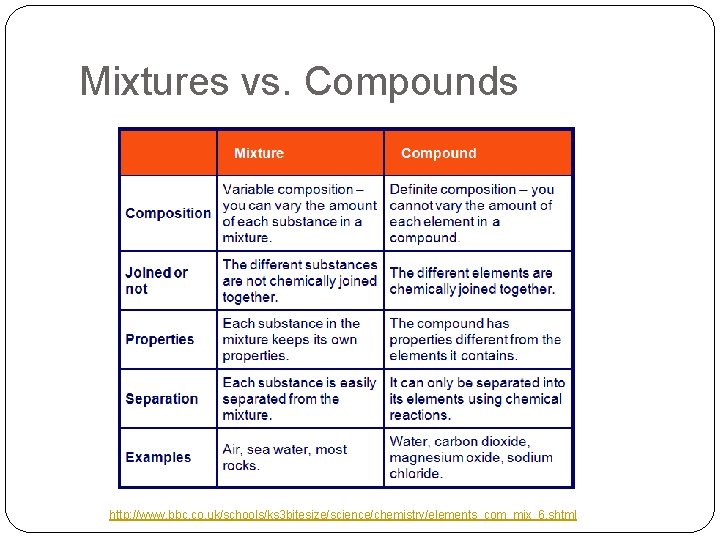
Mixtures vs. Compounds http: //www. bbc. co. uk/schools/ks 3 bitesize/science/chemistry/elements_com_mix_6. shtml

Can you identify the following? You will be shown a series of photos. Tell if each photo represents an item composed of an element, compound, or mixture. Review: An element contains just one type of atom. A compound contains two or more different atoms joined together. A mixture contains two or more different substances that are only physically joined together, not chemically. A mixture can contain both elements and compounds.

Element, Compound, or Mixture? Rocks

Element, Compound, or Mixture? Rocks

Element, Compound, or Mixture? Copper

Element, Compound, or Mixture? Copper

Element, Compound, or Mixture? Jelly Beans

Element, Compound, or Mixture? Jelly Beans

Element, Compound, or Mixture? Table Sugar

Element, Compound, or Mixture? Table Sugar

Element, Compound, or Mixture? Diamond

Element, Compound, or Mixture? Diamond

Element, Compound, or Mixture? Tea

Element, Compound, or Mixture? Tea

Element, Compound, or Mixture? Salt

Element, Compound, or Mixture? Salt

Element, Compound, or Mixture? Neon Gas

Element, Compound, or Mixture? Neon Gas

Element, Compound, or Mixture? Salad

Element, Compound, or Mixture? Salad

Element, Compound, or Mixture? Pure Water

Element, Compound, or Mixture? Pure Water

Element, Compound, or Mixture? Aluminum

Element, Compound, or Mixture? Aluminum

Element, Compound, or Mixture? Lemonade

Element, Compound, or Mixture? Lemonade

Element, Compound, or Mixture? Silver

Element, Compound, or Mixture? Silver

Element, Compound, or Mixture? Sand

Element, Compound, or Mixture? Sand


Notes Detailed notes are located at: http: //www. middleschoolscience. com/elementscompounds-mixtures-notes-isn. pdf Flow Chart: http: //www. middleschoolscience. com/matter-flow-chartisn. pdf