Classifying Matter Classifications Matter can be classified as

Classifying Matter

Classifications Matter can be classified as an element, compound or mixture n Elements- substances consisting of entirely the same atom. n Compounds- substances consisting of entirely the same molecule. n Mixtures- elements and/ or compounds next to each other n

Elements There are 90 naturally occurring atoms on Earth n about only 40 of those can be found naturally in elemental form n Hydrogen, copper, gold, magnesium, lead, oxygen, nitrogen, helium, etc. n Elements are represented by a 1 -2 letter symbol n u The first must always be a capital letter, and the second (if present) is lower case.

Common element symbols n n n n oxygenirongoldchlorinehydrogencoppercarbonsilver- n O Fe n Au n n Cl H n Cu n C Ag n n *notice, that this is not written as FE, make that 2 nd letter lower case not a small capital. A complete alphabetical list is located on pg. 22 of your book

We will not have to memorize the entire periodic table However you will be responsible to know all element symbols with an atomic number 1 -36. (Hydrogen to Krypton) n and Silver (Ag), Gold (Au), Mercury (Hg), Tin (Sn), Iodine (I), Uranium (U), Plutonium (Pu), and Lead (Pb) n You don’t have to remember where they go on the periodic table or their information, only the symbol and name. n

Compounds -substances made up entirely of the same molecule. n molecule- 2 or more atoms bonded together. n these are represented by chemical formulas n element symbols and subscript numbers. n H 2 O n hydrogen (2 of them) oxygen n subscript numbers mean there are that many of the atom it is directly behind. n If there is no subscript number then 1 is implied. n water, ammonia, glass, methane and limestone

Here is where capitalization becomes really important n CO 2 n n n Co 2 n n 1 carbon, 2 oxygen carbon dioxide ( a gas) 2 cobalt atoms cobalt is a metal

Some elements can have molecules as their smallest component as long as the molecule is made up entirely of the same atom n The oxygen we breathe is not 1 oxygen atom, it is O 2 n When 2 atoms are joined like in the above case, it is called a diatomic element n The 7 diatomic elements are hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine n

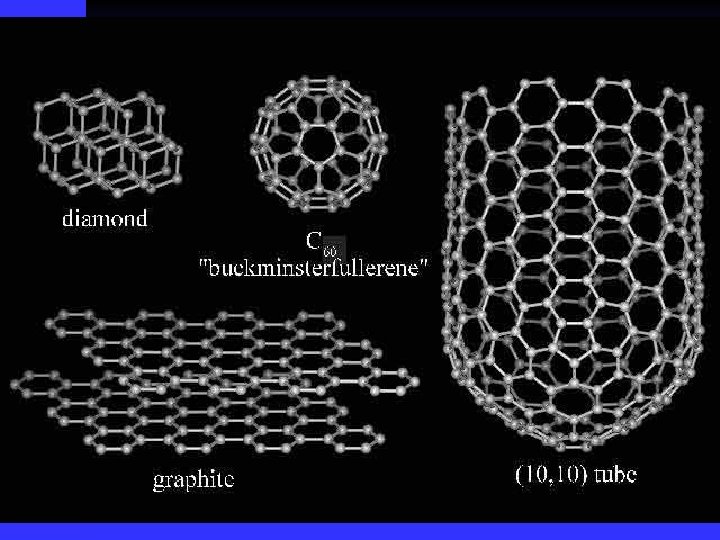
Allotropes allotrope -one of the different molecular forms of an element n oxygen has 2 allotropes n O 2 and O 3 (ozone) n carbon has several allotropes n graphite, diamond, buckyball (found in soot) n

Mixtures compounds and/or elements mixed together but not bonded together n heterogeneous mixture- different throughout or chunky n granite, orange juice with pulp, Italian dressing n homogeneous mixture- even throughout n milk and saltwater n Solution really well mixed homogeneous mixtures n
Breakdown of classification

Separating Mixtures n Mixtures can be separated by chemical or physical means Separating Compounds n compounds can ONLY be separated by chemical means (requires a chemical change)

Separating Elements atom is from the Greek word atomos, meaning not able to be cut. n elements can NOT be separated by chemical or physical means. n The only way to separate an atom is through a nuclear reaction. n
- Slides: 14