Classifying Elements Chapter 6 Section 2 Periodic Table

Classifying Elements Chapter 6 Section 2
Periodic Table • A periodic table is an arrangement of elements in which the elements are separated into groups based on a set of repeating properties. – A periodic table allows you to easily compare the properties of one element to another element.
Squares in the Periodic Table • The periodic table displays the symbols and names of the elements, along with information about the structure of their atoms: • Atomic number and atomic mass • Black symbol = solid; red = gas; blue = liquid • (from the Periodic Table on our classroom wall)

Periodic Table Groups • Each vertical column of the periodic table is called a group, or family. Groups are numbered 1 -18 • Elements within a group have similar chemical and physical properties. – They have similar chemical and physical properties because they have similar electron configurations. • Example: Li = [He] 2 s 1 , Na = [Ne] 3 s 1 each has one electron is the highest energy level
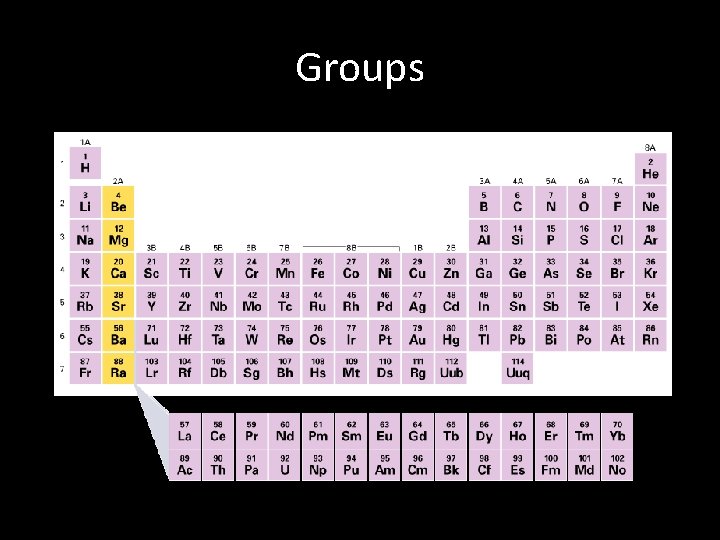
Groups • A Group or Family

Periodic Table Periods • Each horizontal row of the periodic table is called a period. • Within a given period, the properties of the elements vary as you move across it from element to element. • Periods are numbered 1 -7
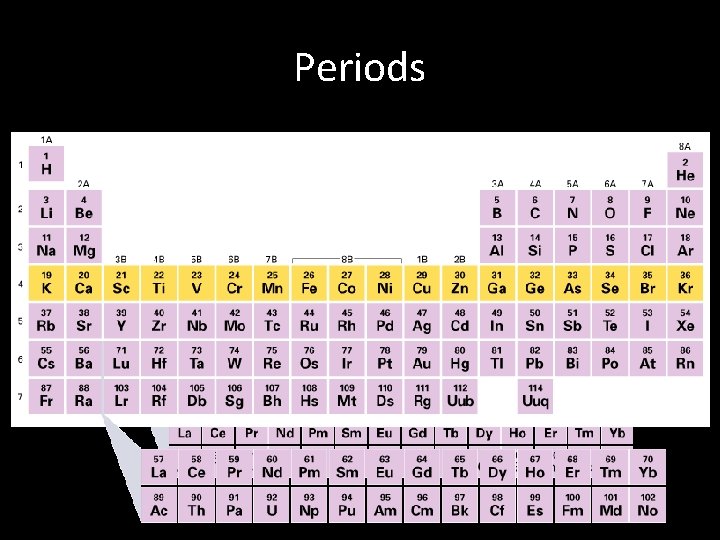
Periods • A Period

Alkali metals • The elements of group 1 of the periodic table except Hydrogen • These elements are extremely reactive, and are never found in nature as pure elements—only found in nature in compounds.
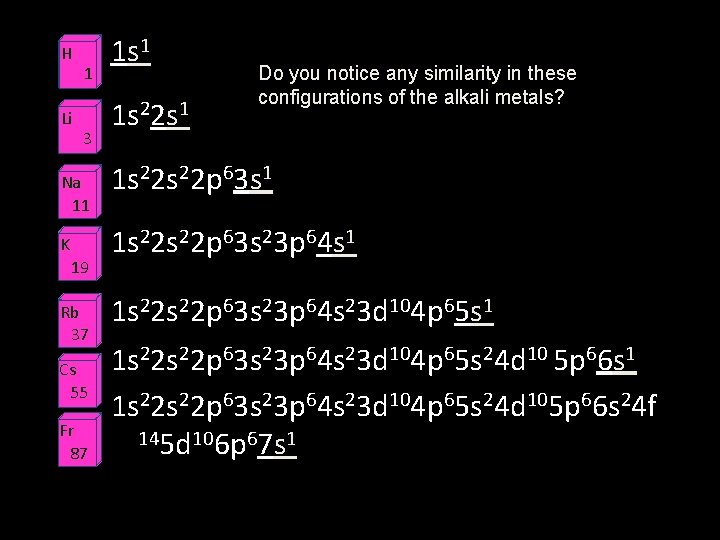
H Li 1 3 Na 11 K 19 Rb 37 Cs 55 Fr 87 1 s 1 1 s 22 s 1 Do you notice any similarity in these configurations of the alkali metals? 1 s 22 p 63 s 1 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10 5 p 66 s 1 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 145 d 106 p 67 s 1

Alkaline Earth Metals • The elements of group 2 of the periodic table • These elements are also very reactive like the alkali metals, just a little bit less reactive. • The alkaline earth metals are also only found in nature as part of a compound.
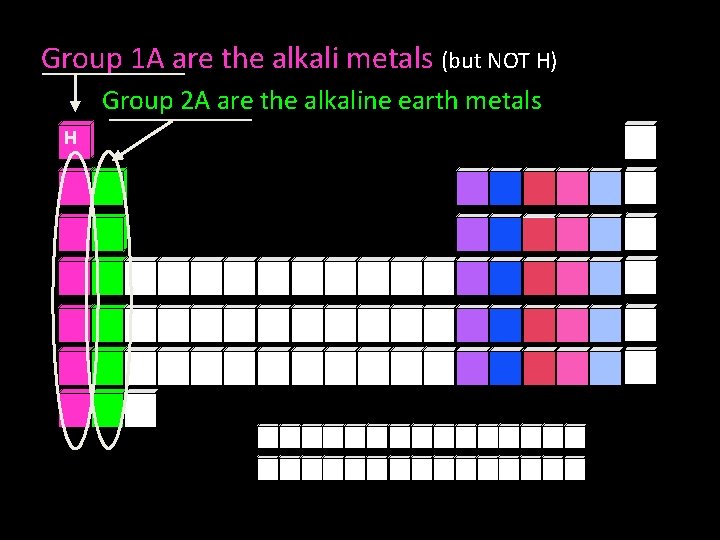
Group 1 A are the alkali metals (but NOT H) Group 2 A are the alkaline earth metals H
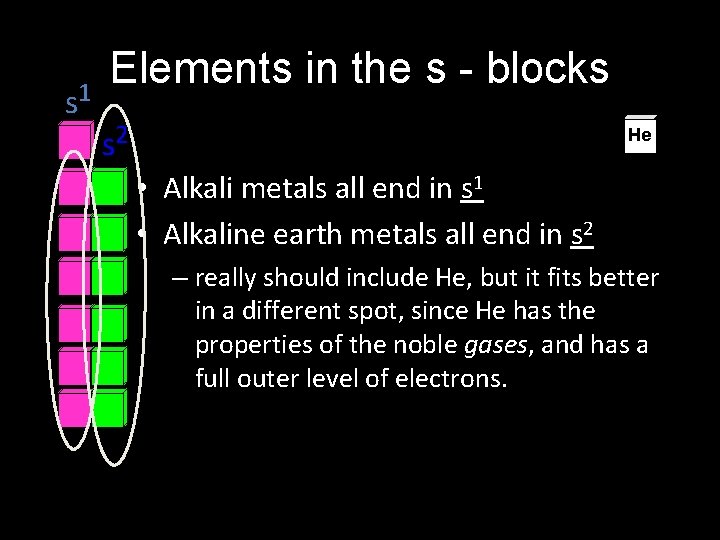
s 1 Elements in the s - blocks s 2 He • Alkali metals all end in s 1 • Alkaline earth metals all end in s 2 – really should include He, but it fits better in a different spot, since He has the properties of the noble gases, and has a full outer level of electrons.

halogens • The nonmetal elements of group 17 of the periodic table • The name halogen comes from the combination of the Greek word hals, meaning salt, and the Latin word genesis, meaning “to be born. ” – Salt forming • The most common halogens are chlorine, bromine, and iodine.
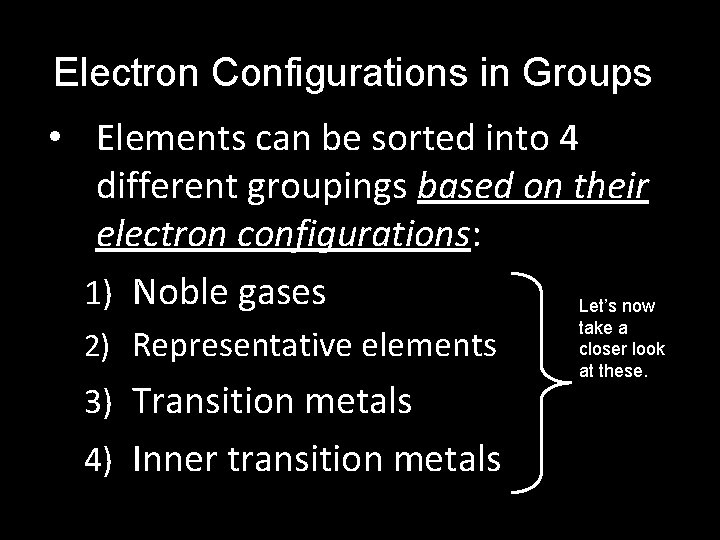
Electron Configurations in Groups • Elements can be sorted into 4 different groupings based on their electron configurations: 1) Noble gases Let’s now 2) Representative elements 3) Transition metals 4) Inner transition metals take a closer look at these.

Noble gases • The elements of group 8 A or 18 of the periodic table • These nonmetals are sometimes called the inert gases because they rarely take part in a reaction. • Noble gases have an electron configuration that has the outer s and p sublevels completely full
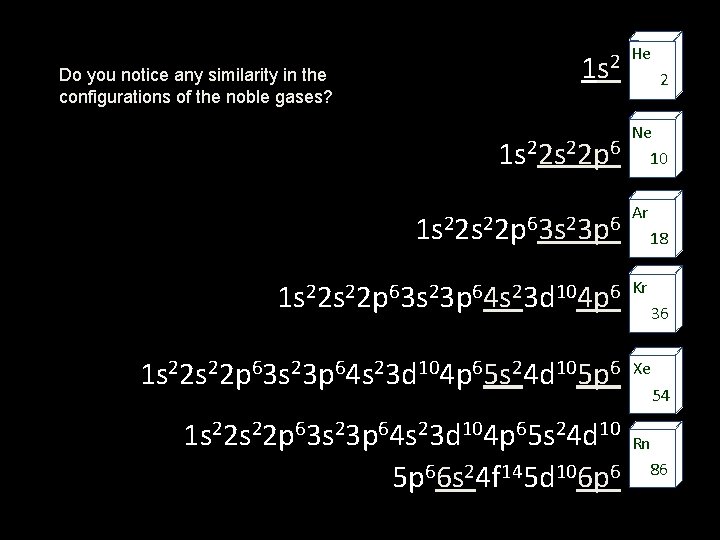
Do you notice any similarity in the configurations of the noble gases? He 2 1 s 1 s 22 p 2 Ne 6 10 1 s 22 p 63 s 23 p 6 Ar 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 Kr 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 6 Xe 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10 5 p 66 s 24 f 145 d 106 p 6 18 36 54 Rn 86
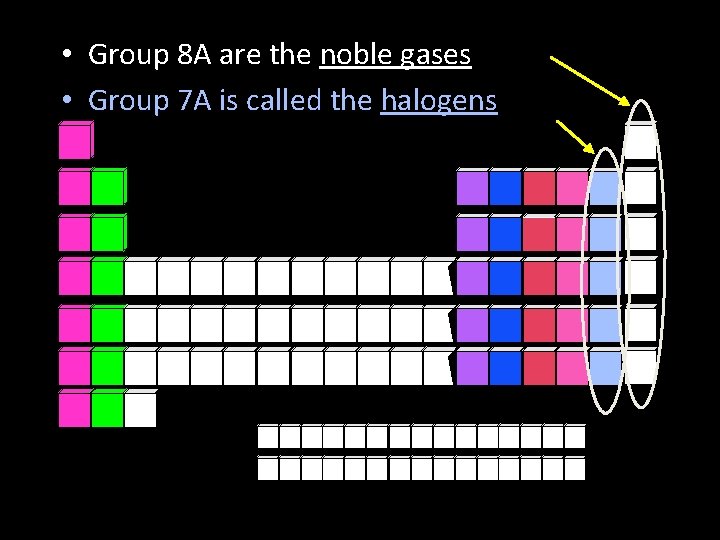
• Group 8 A are the noble gases • Group 7 A is called the halogens

Representative elements • The elements of groups 1, 2, and 13 through 18 or 1 A-7 A of the periodic table • Display wide range of properties, thus a good “representative” • Some are metals, or nonmetals, or metalloids; some are solid, others are gases or liquids • Their outer s and p electron configurations are NOT filled
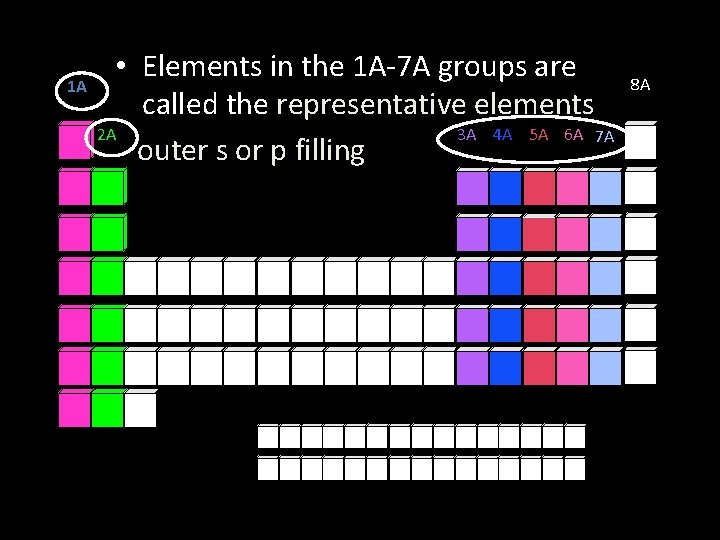
1 A • Elements in the 1 A-7 A groups are called the representative elements 2 A 3 A 4 A 5 A 6 A 7 A outer s or p filling 8 A

Transition metals • The elements of groups 3 through 12 or the B columns of the periodic table • Electron configuration has the outer s sublevel full, and is now filling the “d” sublevel • A “transition” between the metal area and the nonmetal area • Examples are gold, copper, silver
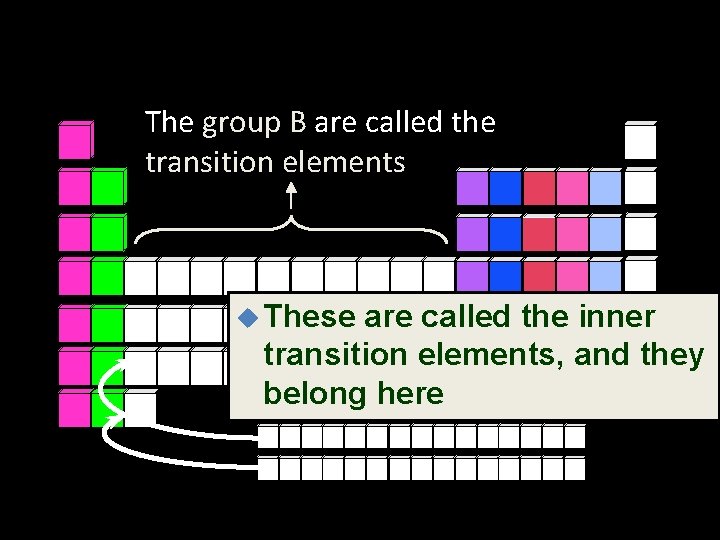
The group B are called the transition elements u These are called the inner transition elements, and they belong here
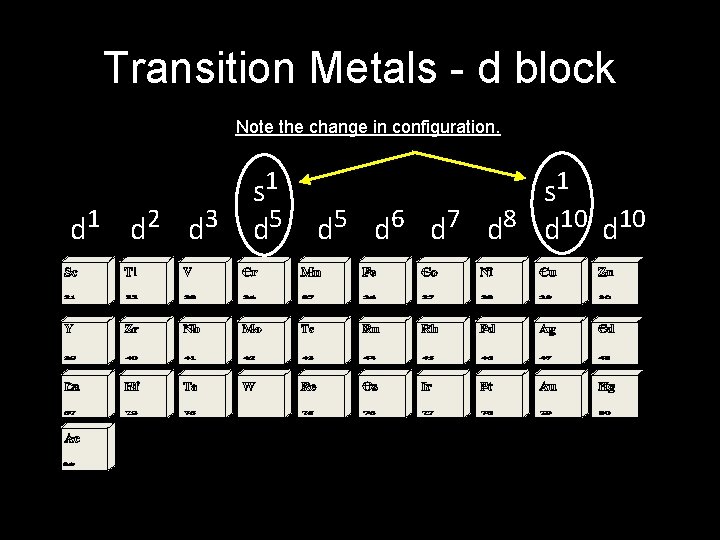
Transition Metals - d block Note the change in configuration. d 1 d 2 d 3 s 1 d 5 d 6 d 7 d 8 d 10
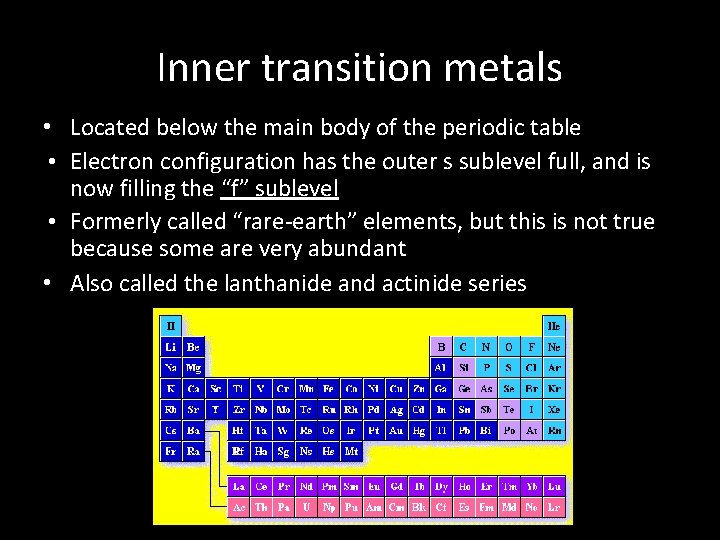
Inner transition metals • Located below the main body of the periodic table • Electron configuration has the outer s sublevel full, and is now filling the “f” sublevel • Formerly called “rare-earth” elements, but this is not true because some are very abundant • Also called the lanthanide and actinide series
- Slides: 23