Classifying Elements Atomic Number Symbol Atomic Mass Periodic
Classifying Elements • Atomic Number • Symbol • Atomic Mass • • • Periodic Table Elements Metals Nonmetals Alloys Noble Gases
What are symbols? • One or two letters that represent the name of an element. • All of the symbols have either one or two letters. • The first letter of each symbol is a capital letter, and the second letter is a small letter. • No period is used at the end of a symbol.
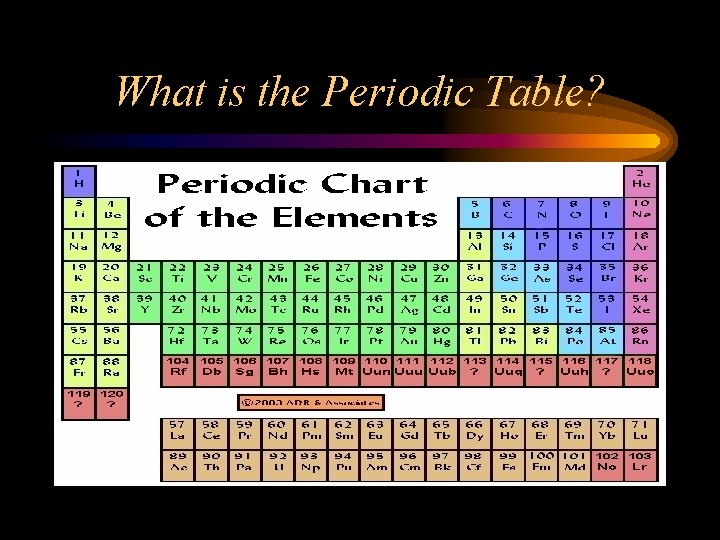
What is the Periodic Table?

Terms to Know Isotopes-one of a group of atoms of an element with the same number of protons but different numbers of neutrons. Deuterium-an isotope of hydrogen that has one proton and one neutron. Tritium-an isotope of hydrogen that has one proton and two neutrons. Atomic mass-the average mass of all the isotopes of a particular element.
Continued terms Family-a group of elements with similar properties, arranged together in a column of the periodic table Metal-one of a group of elements that is usually solid at room temperature, often shiny, and carries heat and electricity well. Alloy-a mixture of two or more metals. Nonmetal-a group of elements with properties opposite to those of metals.

Metal Facts • Most metals are solid at room temperature. • Most metals can be polished to look shiny. • The shape of a metal can be changed. For example, aluminum can be pounded into a thin foil without being broken. Copper is often stretched into very thin wires. • Electricity and heat travel well through metals.

Nobel Gases • One of a group of elements made up of gases that do not combine with other materials under ordinary conditions. Neon is an example of a noble gas. • Inert- a description of an element whose atoms do not react in nature with atoms of other elements.

• Alkali - group 1 – Metals/Very reactive – most reactive metals – Have only 1 electron in outer level – Low density

• Alkaline-Earth (group 2) – Metals/Very reactive (but less than alkali) – have 2 outer level electrons – Higher density than alkali

• Transition Group 3 -12 – Metals/ Tend to be shiny – Tend to conduct thermal energy and electric current well

• Carbon – Nonmetal – several natural forms (diamond/soot) – No life can exist without carbon • Living things, proteins, fats, etc.
• Oxygen – 20% of air you breathe

• Noble Gases – Light bulbs

• Hydrogen – Properties don’t match the properties of any single group – Colorless odorless gas

• Periodic law – States that chemical and physical properties of elements are periodic functions of the elements atomic numbers. – This is WHY? ! – • Elements in vertical groups share similar properties
- Slides: 15