Classifying Chemical COMPOUNDS Acids Bases and Salts Classifying

Classifying Chemical ______ COMPOUNDS Acids, Bases and Salts

Classifying Compounds • We already know how to classify compounds based on the type of bond they form. Ionic: electrons transferred, ions made. (metal + non-metal) Covalent: electrons shared. (non-metal + nonmetal) • We can also classify compounds into many other categories Acids Bases Salts Organics

Today’s Objectives q Be able to distinguish acids and bases by looking at their: q Properties q. Chemical formulas q. Learn about the p. H scale q. How it is related to Acids and Bases q. Its significance q. How we use it Textbook Chapter 8 pages 205 -206

Acids and Bases • Some Common Acids: ▫ Citrus Juice-Orange, Lemon and Lime ▫ Vinegar ▫ Tomatoes Anything with a SOUR taste. • We often add acids to our foods to improve taste and help us to absorb nutrients. CAUTION!!! ▫ Many Acids are CORROSIVE which means they can burn your skin. ▫ Never attempt to identify an acid by touch or taste!

Acids and Bases • Some Common Bases: ▫ Eggs ▫ Baking Soda ▫ Soap ▫ Bleach Anything with a BITTER taste and/or a slippery feel. • CAUTION!!! ▫ Many Bases are CAUSTIC which means they can burn your skin. ▫ Never attempt to identify a base by touch or taste! ***So if we can’t touch or taste compounds how do we know if they are acids or bases?
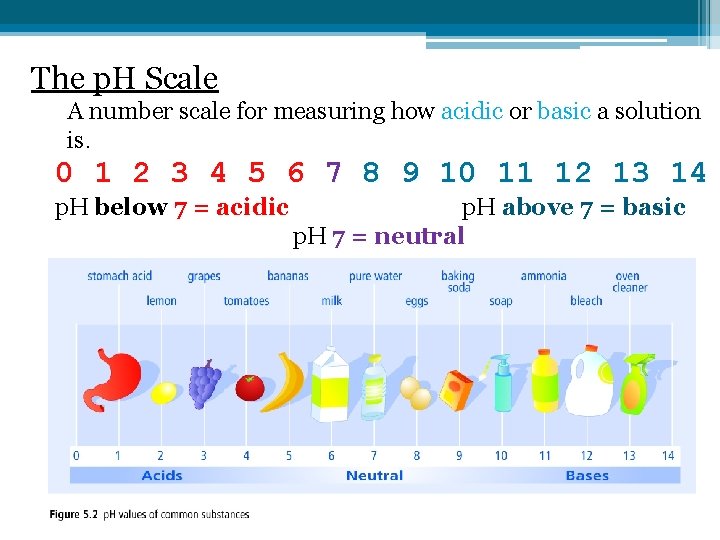
The p. H Scale A number scale for measuring how acidic or basic a solution is. 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 p. H below 7 = acidic p. H above 7 = basic p. H 7 = neutral

What Do the Numbers Mean? ▫ Each decrease of 1 on the p. H scale indicates 10 X more acidic. For example, p. H 4 is 10 x more acidic than p. H 5 p. H 3 is 1000 x more acidic than p. H 6 3 p. H ____ is 100 x more acidic than p. H 5 p. H 1 is 10 000 000 x more acidic than p. H 14

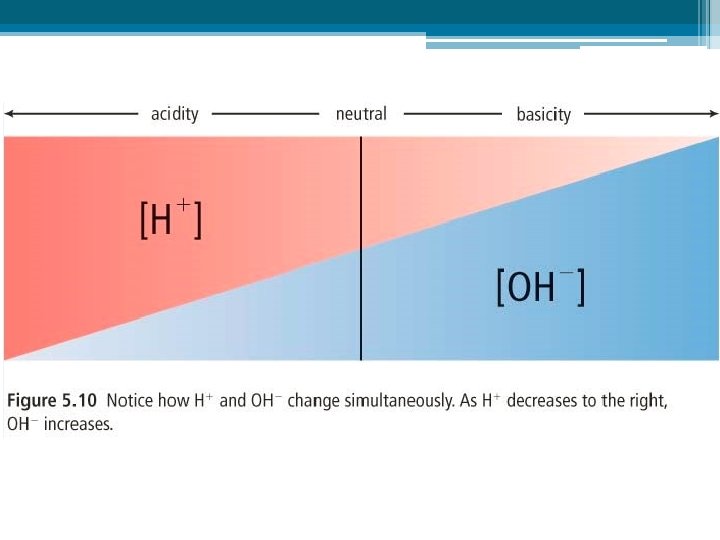
What is p. H really measuring? • The p. H of a solution refers to the concentration of its H+ ions. Remember: CONCENTRATION is the amount of substance (solute) dissolved in a given volume of solution. Phet Square brackets are used to signify concentration, [H+], [OH–] High [H+] = low p. H, very acidic High [OH–] = high p. H, very basic
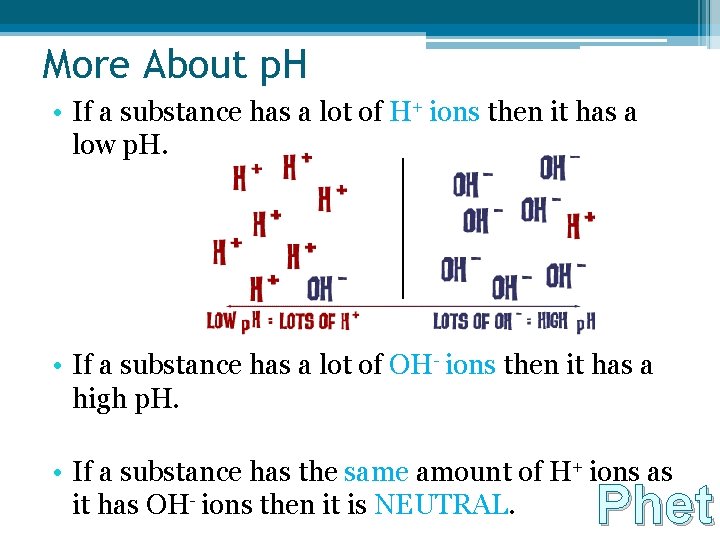
More About p. H • If a substance has a lot of H+ ions then it has a low p. H. • If a substance has a lot of OH- ions then it has a high p. H. • If a substance has the same amount of H+ ions as it has OH- ions then it is NEUTRAL. Phet
p. H Indicators • The p. H of acids and bases cannot be directly determined by sight. ▫ Instead, p. H is measured by other chemicals called indicators or by a p. H meter that measures the electrical conductivity of the solution. • p. H indicators change colour based on the solution they are placed in.
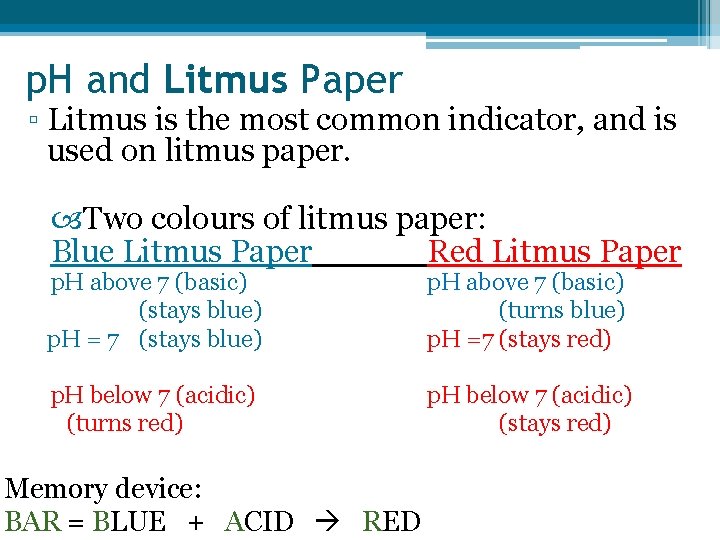
p. H and Litmus Paper ▫ Litmus is the most common indicator, and is used on litmus paper. Two colours of litmus paper: Blue Litmus Paper Red Litmus Paper p. H above 7 (basic) (stays blue) p. H = 7 (stays blue) p. H above 7 (basic) (turns blue) p. H =7 (stays red) p. H below 7 (acidic) (turns red) p. H below 7 (acidic) (stays red) Memory device: BAR = BLUE + ACID RED

Litmus Paper ACID BASE

p. H Probes • A p. H meter uses electrical probes to measure how solutions conduct electricity. We said that acids and bases like to form H+ ions and OH- ions when in solutions and the concentration of these ions will determine a solution’s electrical conductivity.

More About ACIDS • Acids readily react with metals to produce hydrogen gas • We can also identify Acids by looking at their chemical formula. • The chemical formula of an acid usually starts with hydrogen (H_). • Acids with a carbon usually have the C written first. • Acids often behave like acids only when dissolved in water and so they often have the subscript (aq) • Acids conduct electricity because they release hydrogen ions, H+(aq)
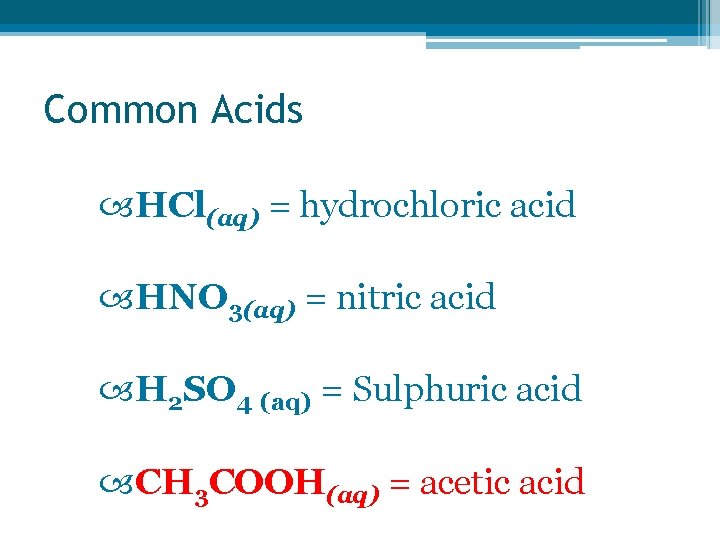
Common Acids HCl(aq) = hydrochloric acid HNO 3(aq) = nitric acid H 2 SO 4 (aq) = Sulphuric acid CH 3 COOH(aq) = acetic acid

More About Bases • We can also identify Bases by looking at their chemical formula. • The chemical formula of a base usually ends with hydroxide (OH). • Bases release hydroxide ions OH–(aq) • Bases often behave like bases only when dissolved in water and so they often have the subscript (aq) • Don’t readily react with metals

Examples of common bases ▫ Na. OH(aq) – Sodium Hydroxide ▫ Mg(OH)2(aq) – Magnesium Hydroxide ▫ Ca(OH)2(aq) – Calcium Hydroxide ▫ NH 4 OH(aq) – Ammonium Hydroxide

ACIDS BASES p. H less than 7, corrosive p. H more than 7, caustic Litmus turns RED Litmus turns BLUE Conduct electricity (when dissolved in water) Chemical Formula starts Chemical Formula ends with H with OH React with metals to produce H gas Do not readily react with metals

Review • We can classify Acids and Bases based on: ▫ Their p. H ▫ Their colour change with indicators ▫ Their conductivity ▫ Their chemical formula ▫ Their reactivity

What About SALTS? • What is a salt? ? ? • Salts are ionic compounds formed when acids and bases react. ▫ Salts are also produced when oxides or carbonates react with acids or when metals react with acids. • Table salt, Na. Cl, is found in sea water, salt lakes or rock deposits. • Na. Cl is only one kind of salt. ▫ A salt is made up of a positive ion from a base and a negative ion from an acid. ▫ Salts are found in many things: In batteries, explosives and fertilizers In multivitamins In many living cells
- Slides: 21