Classification of Polymer Classification based on source Natural
Classification of Polymer * Classification based on source : -Natural polymer : -Synthetic polymer -Modified natural polymer • Classification based on Chemical Natural of polymer : • A-Organic polymer • B-Inorganic polymer • C-Organic –Inorganic polymer

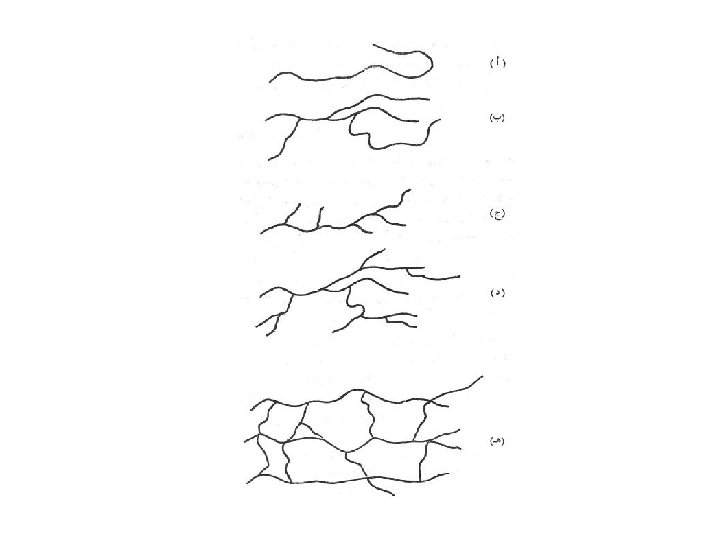
Classification based on the structural shape of molecules: • Linear Polymers • Branched polymers • Crosslinked polymers

Classification based on the reaction mechnisim: • Condensation Polymerization – two or more different organic molecules react to produce a chain made up of combinations of starting molecules that releases a by-product, such as water • Addition Polymerization – one or more organic molecules containing a shared electron bond, i. e. double bond, is split by the action of an initiator – produces unpaired electrons on the starting molecule which subsequently bond with surrounding molecules of the same type by addition until a long chain is produced
Classification based on technological aspects: • Thermoplastic Polymers – behave in a plastic, ductile behavior – formed at elevated temperatures, cooled, and then reheated and reformed into a different shape – final basic structure or properties of the polymer is unchanged during processing • Thermosetting Polymers – network polymers often formed by condensation – general stronger than thermoplastics – polymer cannot be reprocessed because part of the molecule (i. e. the by-product) has left the material • Elastomers – intermediate physical behavior – ability to elastically deform at large strains without permanently changing shape
Other ways to classify polymers. – By chemical functionality (e. g. polyacrylates, polyamides, polyethers, polyurethanes…). – Vinyl vs. non-vinyl polymers. – By polymerization methods (radical, anionic, cationic…). – Etc…

The Texture of Polymers Intermolecular Forces in Polymers Covalent Bonds Secondary Forces Inorganic Polymers

Semi Organic Polymers Coordination Bonds Ionic Bonds Secondary Forces Van der Waals Inter Molecular Forces

Dipole Forces
- Slides: 9