Classification of Matter Unit 9 Lecture 1 Matter








- Slides: 11

Classification of Matter Unit 9 Lecture 1

Matter Has mass (different than weight which is mass due to gravity) Occupies space

Element Pure substance ◦ Made of same type of atoms Only one symbol from a periodic table Cannot be separated into simpler substances always has same properties as another pure sample ◦ Example: copper wire

Element Activities Click on my webpage links under Chemistry and choose Periodic Table Elements and Games. ◦ Naming elements ◦ Naming Symbols

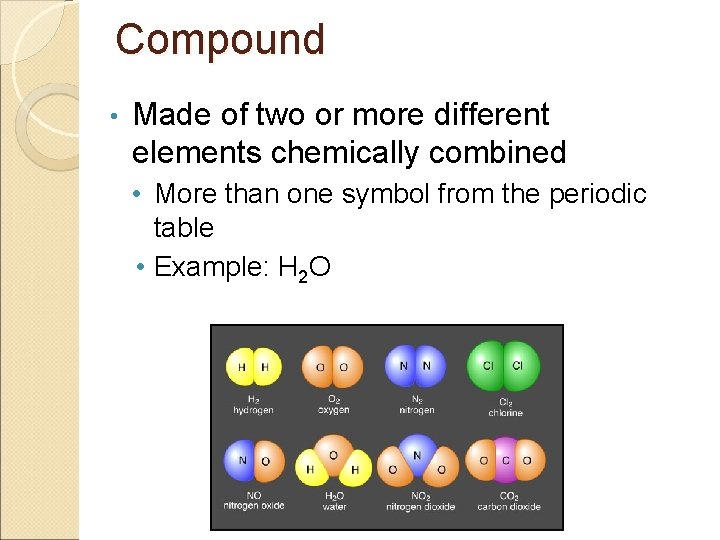
Compound • Made of two or more different elements chemically combined • More than one symbol from the periodic table • Example: H 2 O

Properties of a compound differ from those of the elements that it is made up from ◦ Water: liquid at room temperature; puts out fires ◦ Hydrogen: gas at room temperature; flammable ◦ Oxygen: gas at room temperature; flammable

3. Mixtures • consist of parts that have different properties – Consists of two or more substances that each retain their own individual properties • 3 ways formed: – 2 or more elements mixed together • Mixture of copper and nickel coins – A compound and element mixed together – 2 or more compounds mixed together • Mixture of sugar and salt

Properties of Mixtures Mixture retains properties of each of the different parts of the mixture Composition of mixture varies Example: Bag of fruit snacks

Types of Mixtures Homogenous ◦ sample from one part of mixture has the same composition as a sample from any other part of the mixture Example: Ketchup, perfume, shampoo

Solutions Homogenous 2 mixture parts: ◦ Solute: substance that gets dissolved ◦ Solvent: substance in which the solute gets dissolved Example: Salt water ◦ Salt = solute ◦ Water = solvent

Heterogeneous ◦ Sample of matter that has parts with different compositions Throw salt on top of sugar – not equal composition throughout mixture Example: italian dressing