Classification of Matter Stuff of which all materials
















- Slides: 33

Classification of Matter Stuff of which all materials are made: anything that has mass and takes up space.

Matter Ø All matter is composed of atoms Ø Atoms are extremely small building blocks of matter Ø Atoms cannot be broken down into smaller pieces by chemical means Ø Atoms are the smallest distinct units in a sample of matter

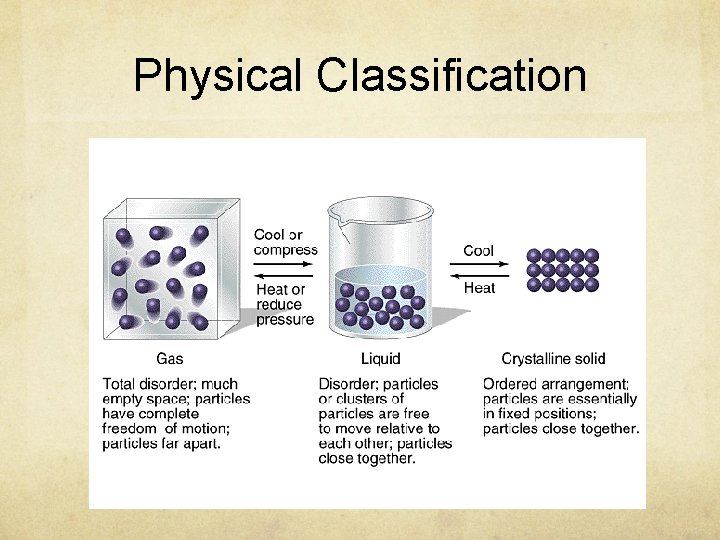
Physical Classification

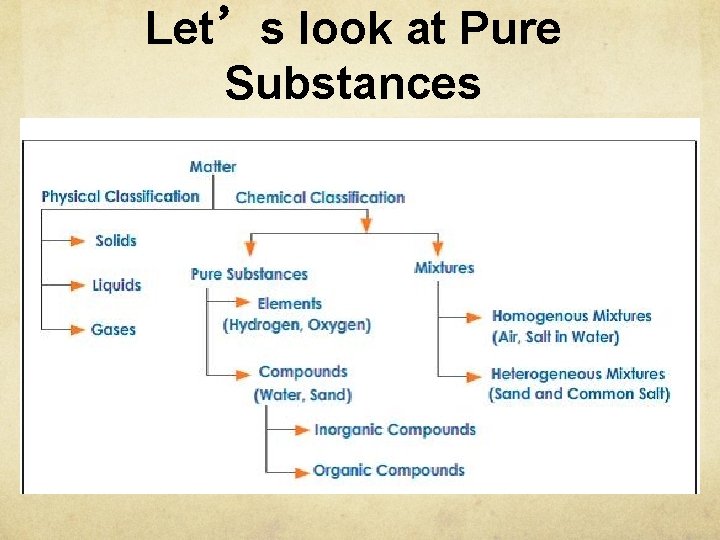
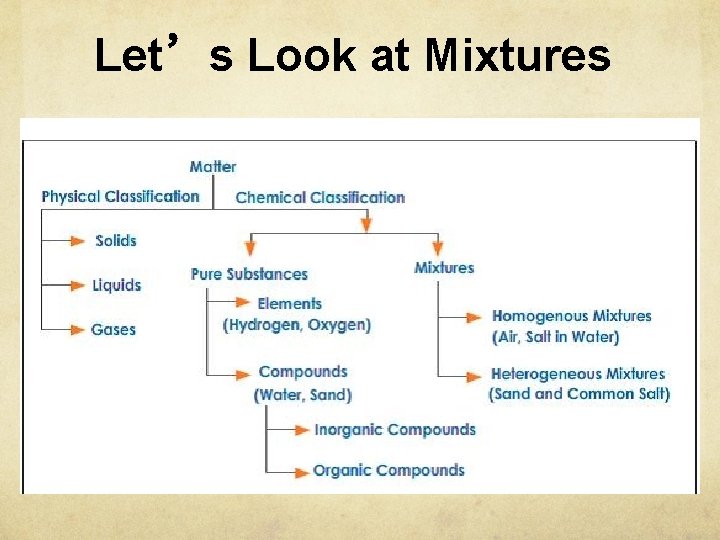
Let’s look at Pure Substances

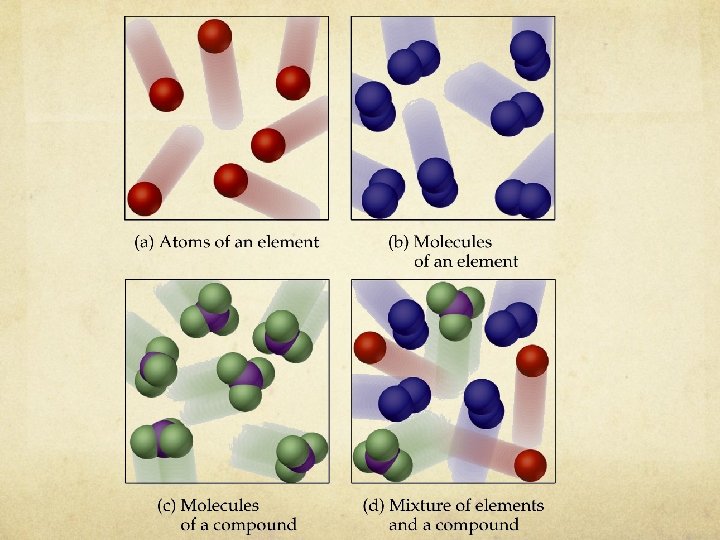
Pure Substances Element Pure substance composed of one type of atom cannot be decomposed into other substances. Represented by one or two letter symbol EX: copper wire (Cu), aluminum foil (Al)

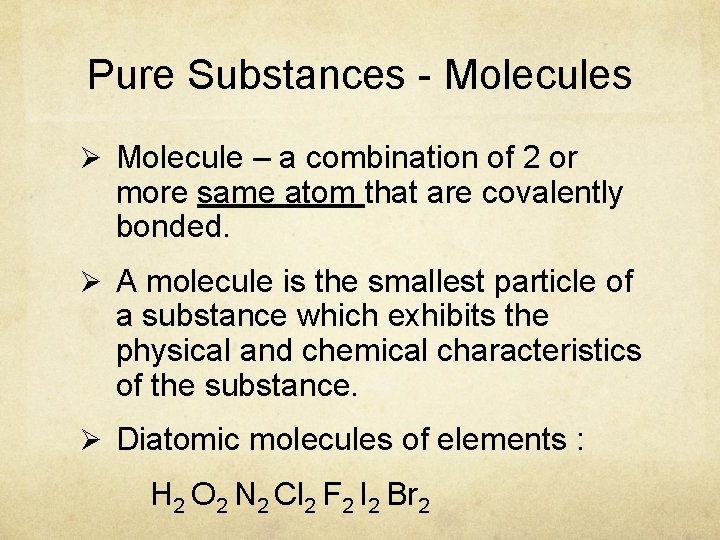
Pure Substances - Molecules Ø Molecule – a combination of 2 or more same atom that are covalently bonded. Ø A molecule is the smallest particle of a substance which exhibits the physical and chemical characteristics of the substance. Ø Diatomic molecules of elements : H 2 O 2 N 2 Cl 2 F 2 I 2 Br 2

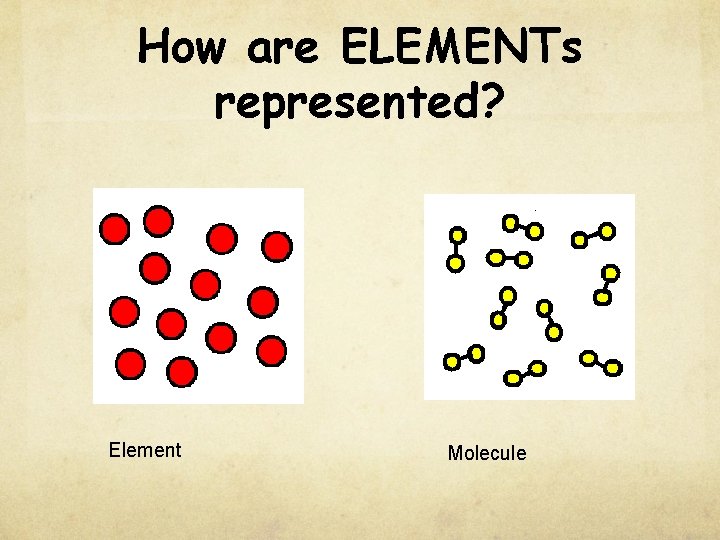
How are ELEMENTs represented? Element Molecule

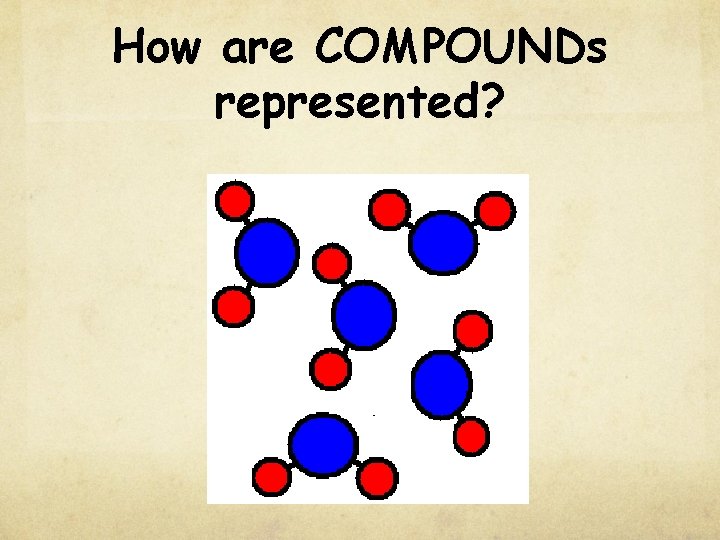
Pure Substances Compound composed of two or more elements chemically combined in a fixed ratio properties differ from those of individual elements Represented by a chemical formula EX: water (H 2 O) table salt (Na. Cl)

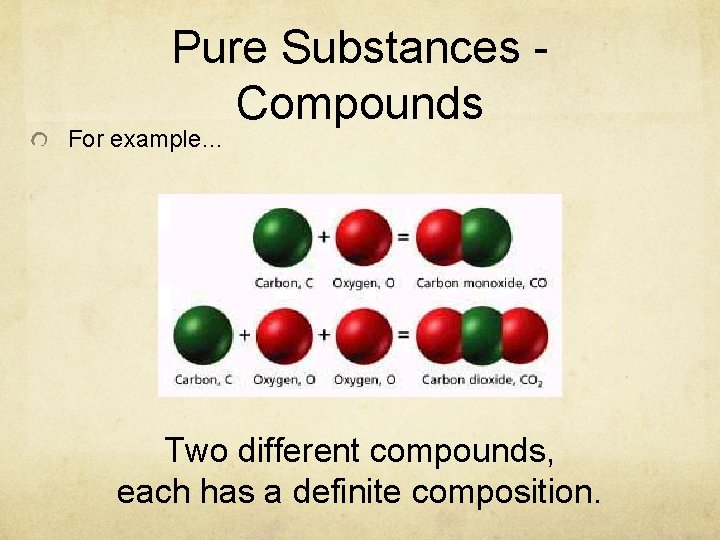
Pure Substances Compounds For example… Two different compounds, each has a definite composition.

Pure Substances Compounds a compound of 2 or more different elements bonded together in a fixed proportion. H 2 O Ca. SO 4 CO 2 HBR Na 2 O H 2 CO 3 KOH

How are COMPOUNDs represented?

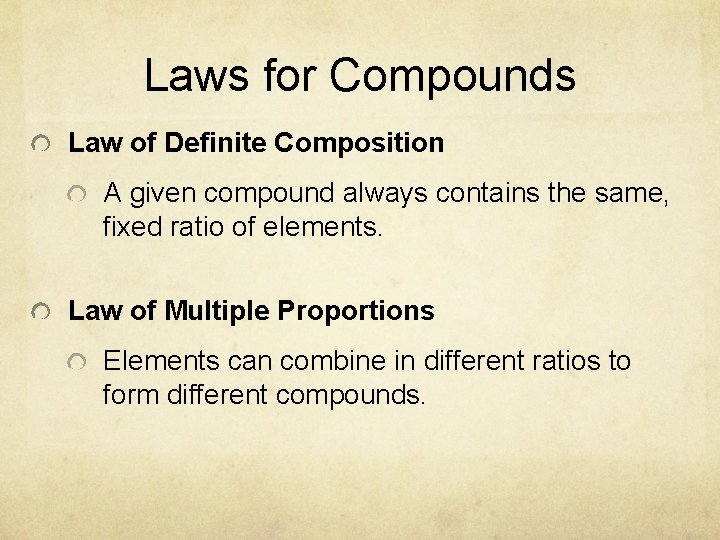
Laws for Compounds Law of Definite Composition A given compound always contains the same, fixed ratio of elements. Law of Multiple Proportions Elements can combine in different ratios to form different compounds.

Pure Substances Compounds Slight differences in combinations of atoms can have large difference in properties H 2 O - water H 2 O 2 – hydrogen peroxide C 2 H 6 O – ethanol, drinkable C 2 H 6 O 2 – ethylene glycol, poisonous

Pure Substances Same kind of particles throughout Compounds Can be decomposed into simpler substances by chemical changes, always in a definite ration Elements Cannot be decomposed into simpler substances by chemical changes

Let’s Look at Mixtures

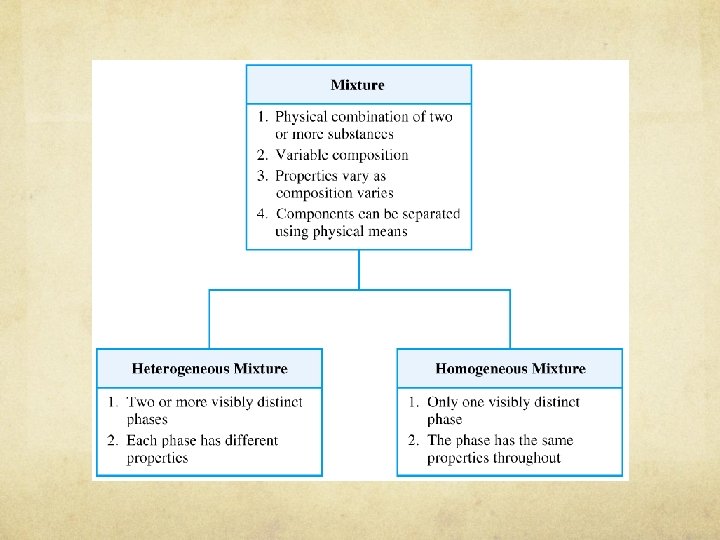
Mixtures are two or more substances that are physically combined. Mixtures do not have a fixed composition Mixtures do not have constant boiling points or melting points Variable composition Components retain their characteristic properties

Mixture May be separated into pure substances by physical methods Mixtures of different compositions may have widely different properties.


Mixtures can be described by how uniform they are throughout. Heterogeneous Homogeneous


Mixtures - Heterogeneous mixture Two or more phases (with same or different physical states) Each phase has different properties Not uniform throughout Examples: oil and water, sand

Mixtures – Homogenous solution Homogenous mixture One phase – must always be the same Uniform throughout Same composition in a sample Examples: sugar, salt water, rubbing alcohol

Mixtures - Homogenous Alloys – combination of two or more metals Examples: brass and steel

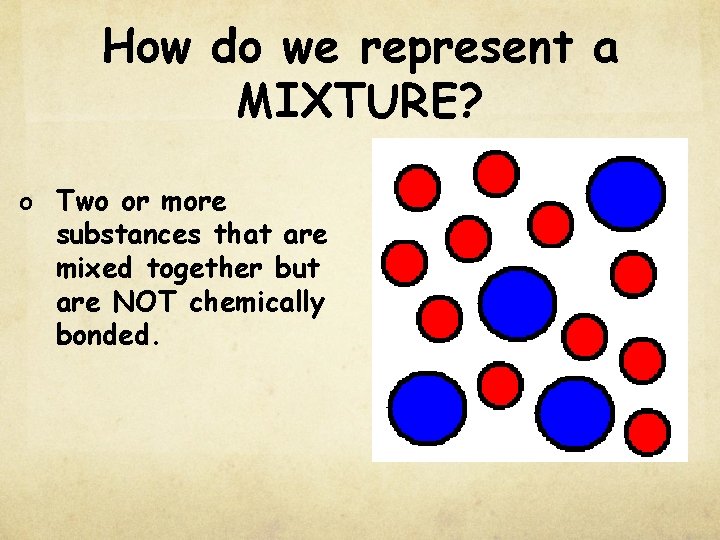
How do we represent a MIXTURE? o Two or more substances that are mixed together but are NOT chemically bonded.

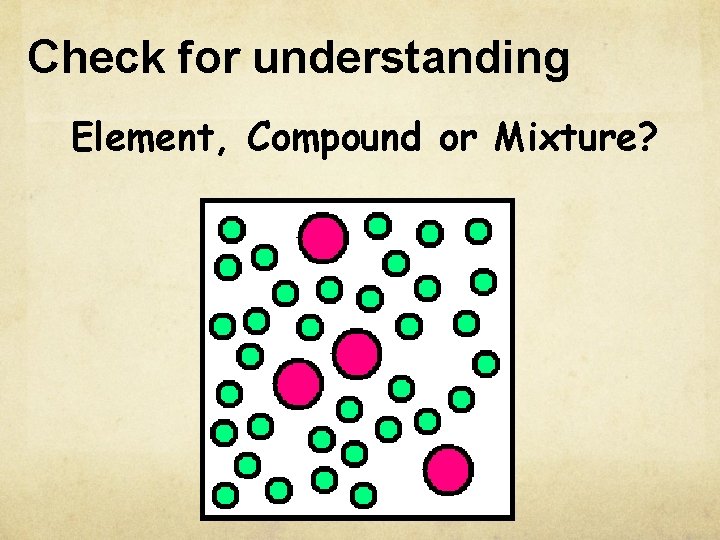
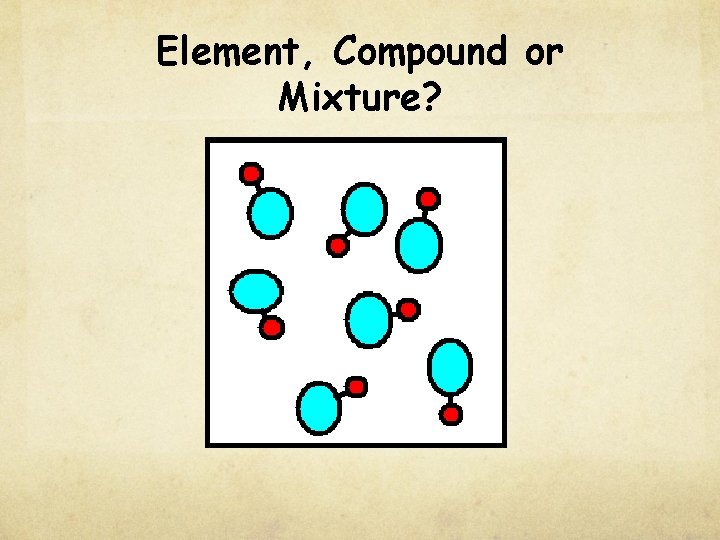
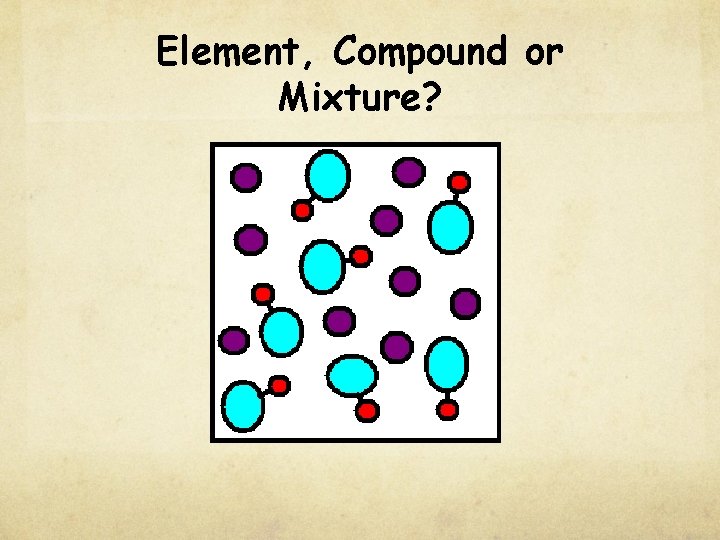
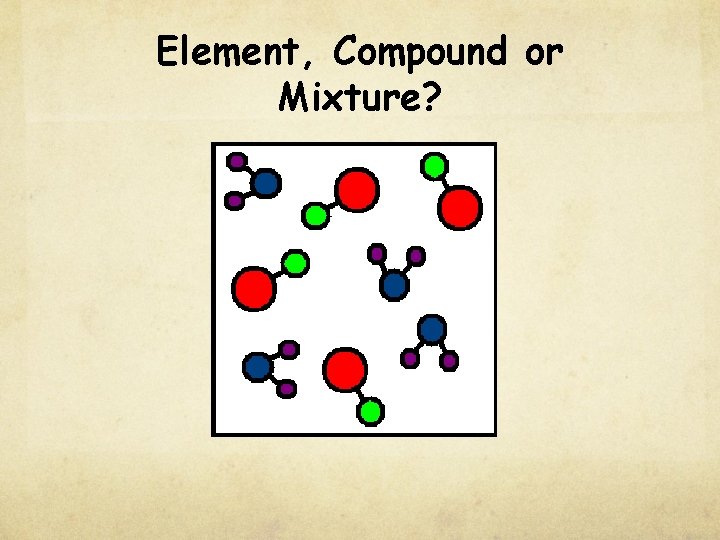
Check for understanding Element, Compound or Mixture?

Element, Compound or Mixture?

Element, Compound or Mixture?

Element, Compound or Mixture?

Check for Understanding Number your papers from 1 to 15. Classify the following as an element, compound, or mixture (heterogeneous or homogeneous). 1. Penny 6. Oxygen 11. Sea 2. Air 7. Sugar 12. Water 3. Al foil 8. Chlorine gas 13. water Graphite 4. Windex 9. Salad dressing 14. Pepper

How did you do? 1. Penny – Mixture Homogenous (zinc and copper solids) 2. Air- Mixture Homogenous (gases) 3. Aluminum foil - Element 4. Windex – Mixture Homogenous 5. Steel – Mixture Homogenous (iron and carbon solids) 6. Oxygen – Element 7. Sugar – Compound (C 6 H 12 O 6)

How did you do? 9. Salad Dressing - Mixture Heterogenous 10. Table salt – Compound (Na. Cl) 11. Sea water – Mixture Homogenous 12. Water – Compound 13. Graphite – Element (Carbon) 14. Pepper – Mixture - heterogeneous 15. Paint – Mixture - heterogeneous

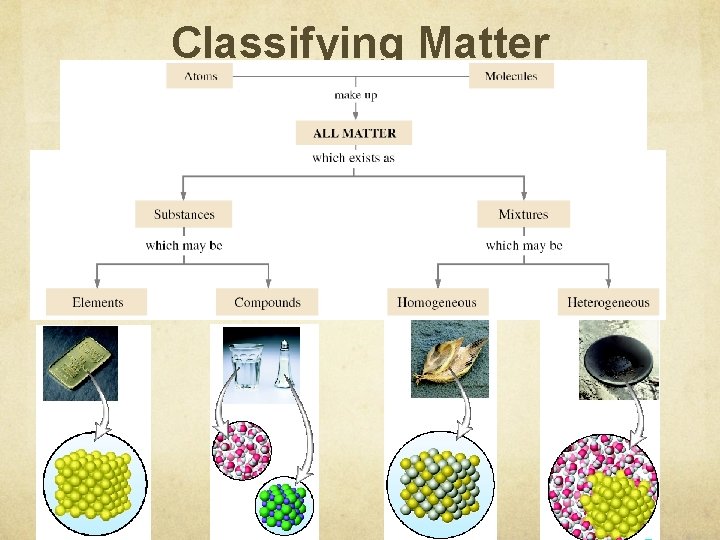
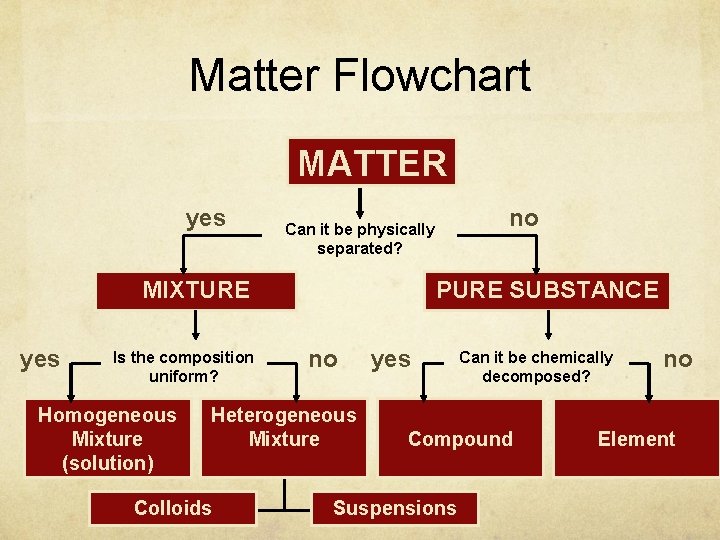
Classifying Matter

Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions no Element