Classification of Matter States of Matter Physical and
























































- Slides: 115

Classification of Matter States of Matter Physical and Chemical Properties Physical and Chemical Changes 1

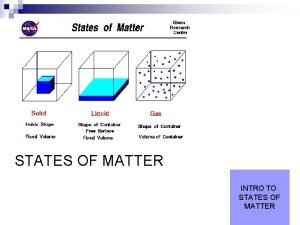
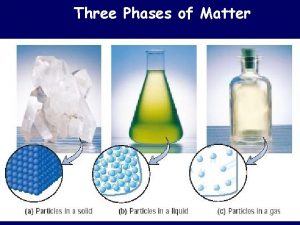
Classification of Matter • Matter is anything that has mass and occupies space. • We can classify matter based on whether it’s solid, liquid, or gas. 2

Define Atoms- Extremely small building blocks of matter Ø All matter is composed of atoms Ø Atoms cannot be broken down into smaller pieces by chemical means Ø The smallest distinct units in a sample of matter Elements are made up the same atoms. Ø Elements cannot be decomposed into other substances.

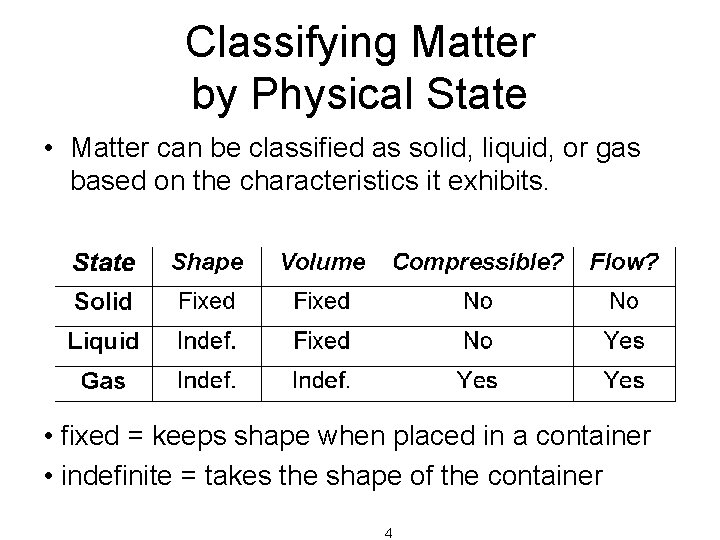
Classifying Matter by Physical State • Matter can be classified as solid, liquid, or gas based on the characteristics it exhibits. • fixed = keeps shape when placed in a container • indefinite = takes the shape of the container 4

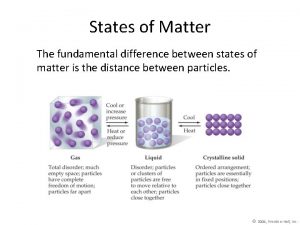
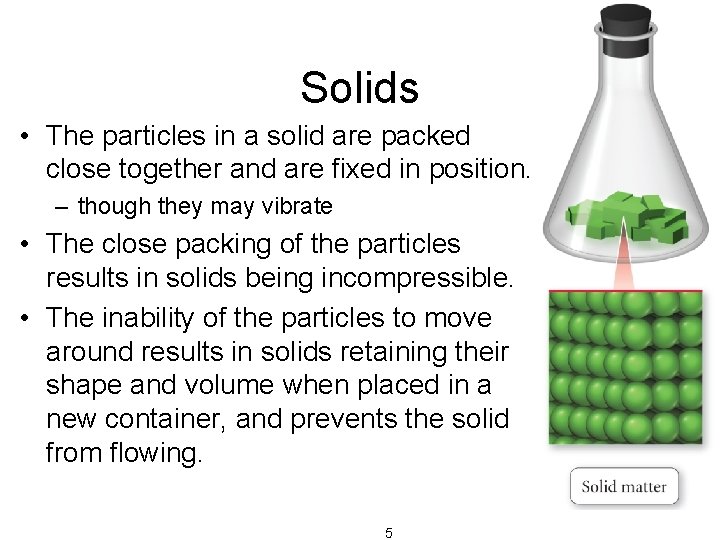
Solids • The particles in a solid are packed close together and are fixed in position. – though they may vibrate • The close packing of the particles results in solids being incompressible. • The inability of the particles to move around results in solids retaining their shape and volume when placed in a new container, and prevents the solid from flowing. 5

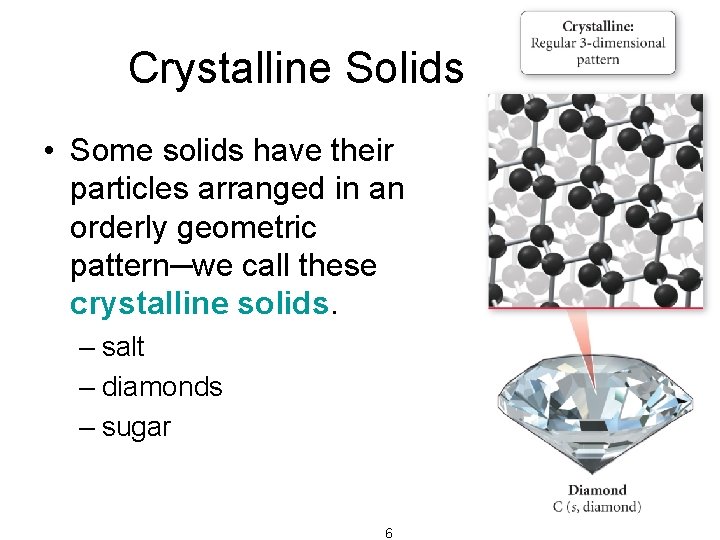
Crystalline Solids • Some solids have their particles arranged in an orderly geometric pattern─we call these crystalline solids. – salt – diamonds – sugar 6

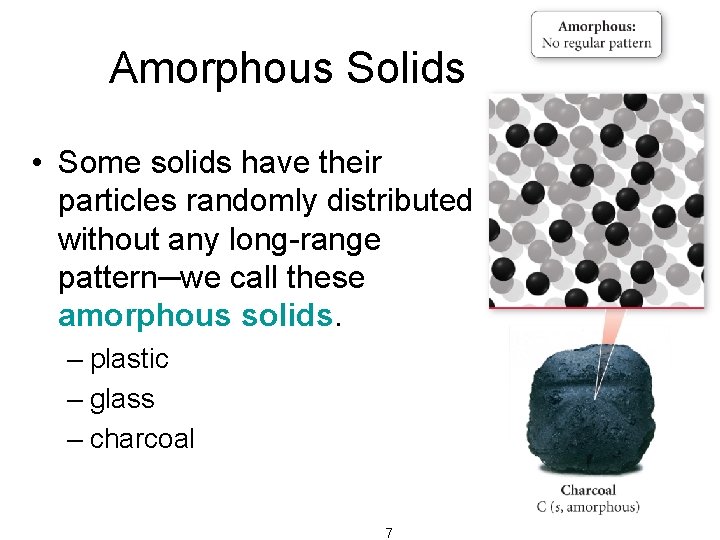
Amorphous Solids • Some solids have their particles randomly distributed without any long-range pattern─we call these amorphous solids. – plastic – glass – charcoal 7

Liquids • The particles in a liquid are closely packed, but they have some ability to move around. • The close packing results in liquids being incompressible. • The ability of the particles to move allows liquids to take the shape of their container and to flow; however, they don’t have enough freedom to escape and expand to fill the container. 8

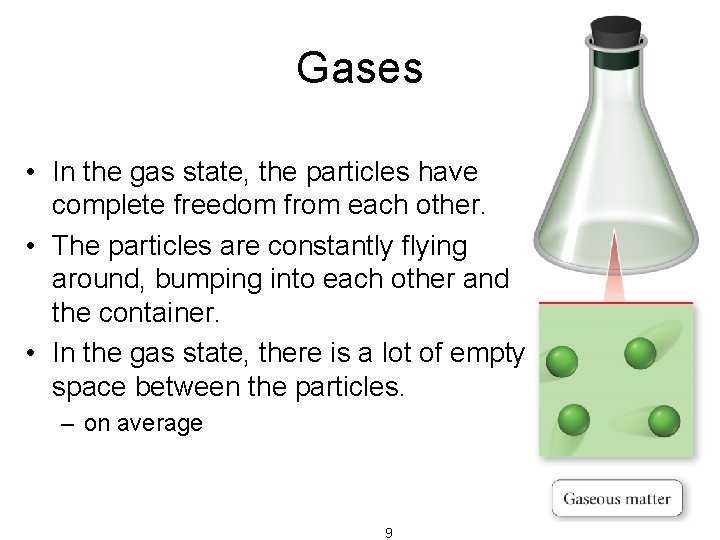
Gases • In the gas state, the particles have complete freedom from each other. • The particles are constantly flying around, bumping into each other and the container. • In the gas state, there is a lot of empty space between the particles. – on average 9

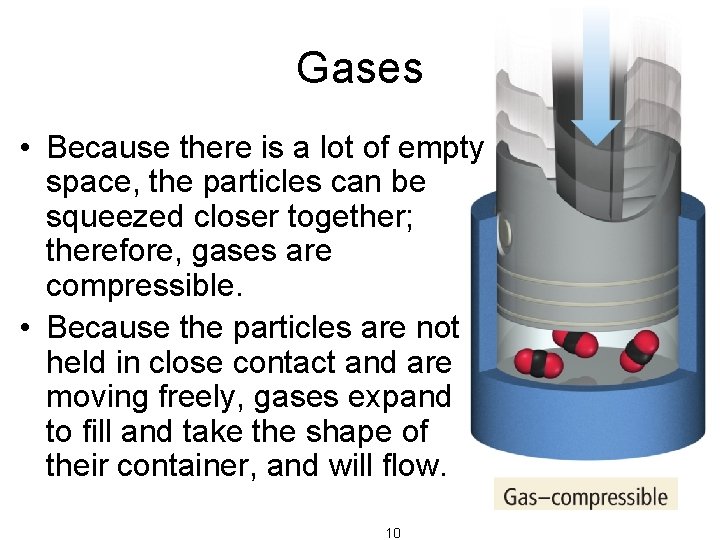
Gases • Because there is a lot of empty space, the particles can be squeezed closer together; therefore, gases are compressible. • Because the particles are not held in close contact and are moving freely, gases expand to fill and take the shape of their container, and will flow. 10

But what happens if you raise the temperature to super-high levels… between 1000°C and 1, 000, 000°C ? Will everything just be a gas?

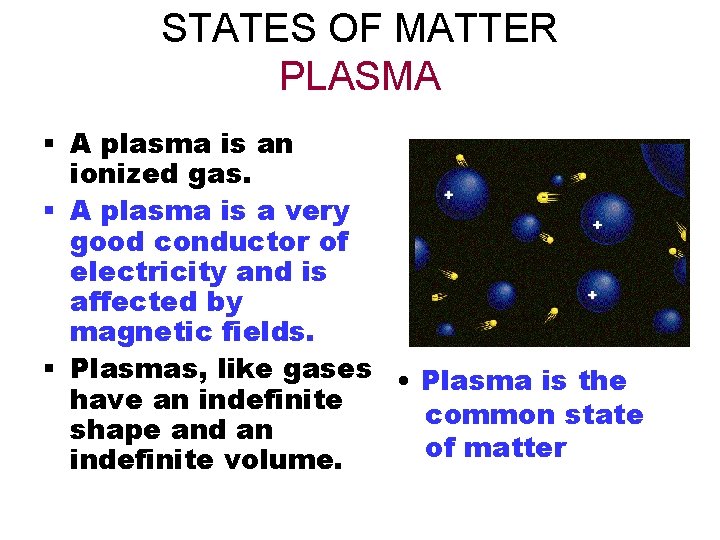
STATES OF MATTER PLASMA § A plasma is an ionized gas. § A plasma is a very good conductor of electricity and is affected by magnetic fields. § Plasmas, like gases • Plasma is the have an indefinite common state shape and an of matter indefinite volume.

STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

Some places where plasmas are found… 1. Flames

2. Lightning

3. Aurora (Northern Lights)

The Sun is an example of a star in its plasma state


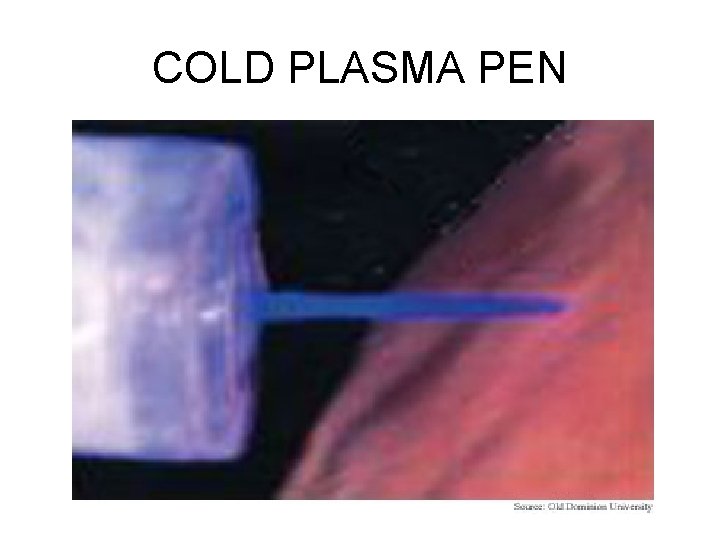
COLD PLASMA

COLD PLASMA PEN

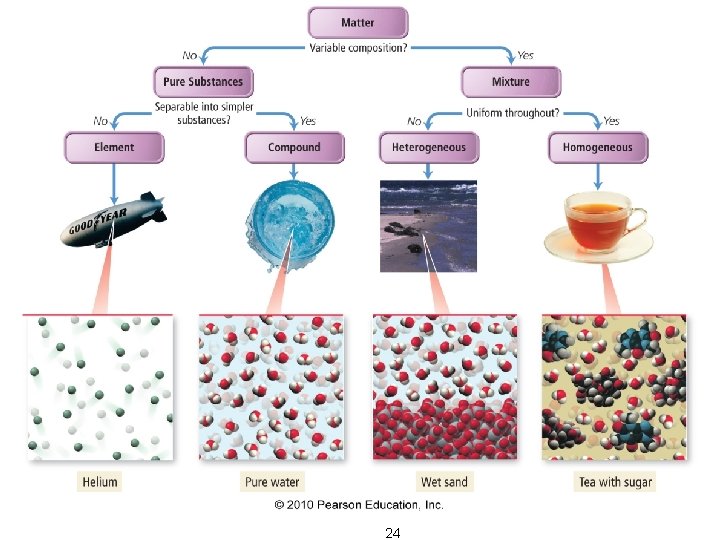
Classifying Matter by Composition • Another way to classify matter is to examine its composition. • composition includes: – types of particles – arrangement of the particles – attractions and attachments between the particles 21

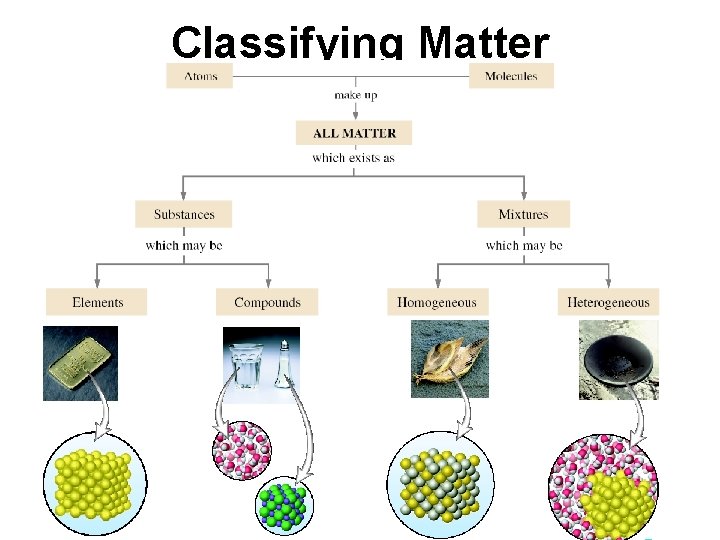
Classifying Matter

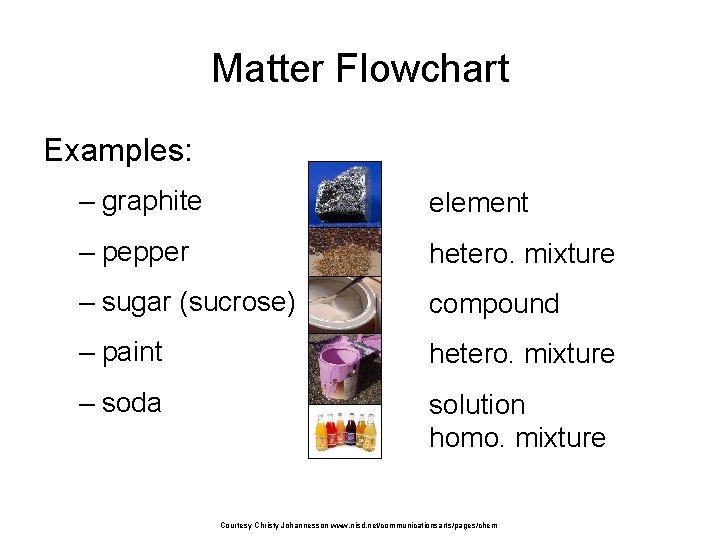
Matter Flowchart Examples: – graphite element – pepper hetero. mixture – sugar (sucrose) compound – paint hetero. mixture – soda solution homo. mixture Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

24

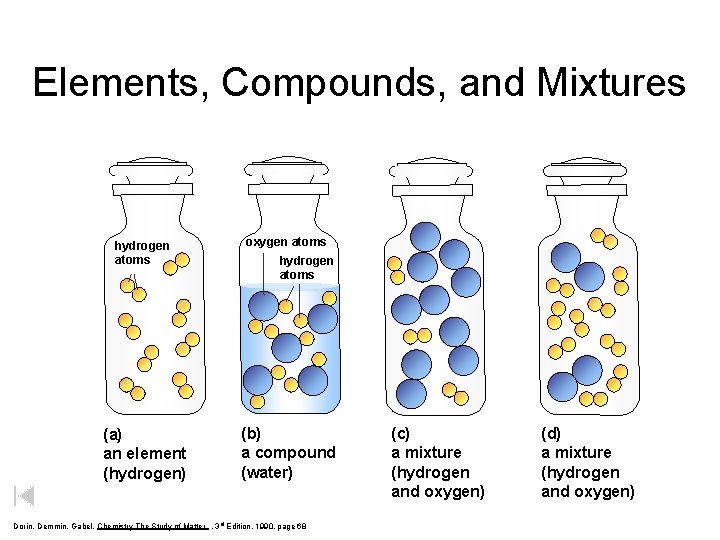
Elements, Compounds, and Mixtures hydrogen atoms oxygen atoms (a) an element (hydrogen) (b) a compound (water) hydrogen atoms Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 68 (c) a mixture (hydrogen and oxygen) (d) a mixture (hydrogen and oxygen)

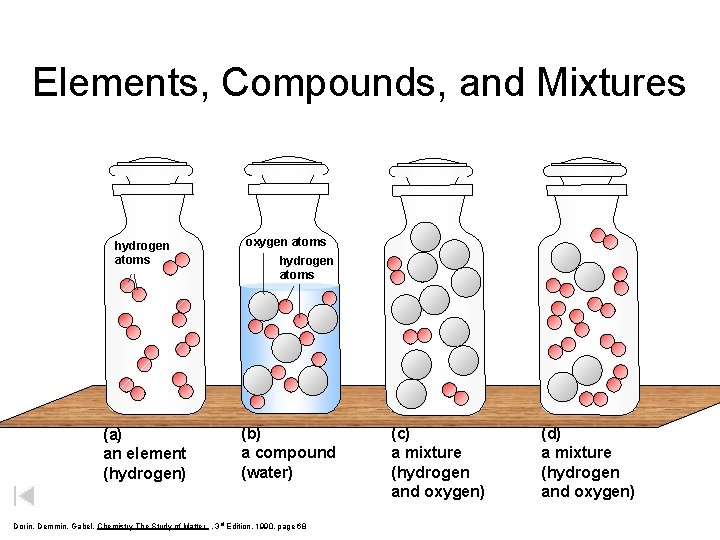
Elements, Compounds, and Mixtures hydrogen atoms oxygen atoms (a) an element (hydrogen) (b) a compound (water) hydrogen atoms Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 68 (c) a mixture (hydrogen and oxygen) (d) a mixture (hydrogen and oxygen)

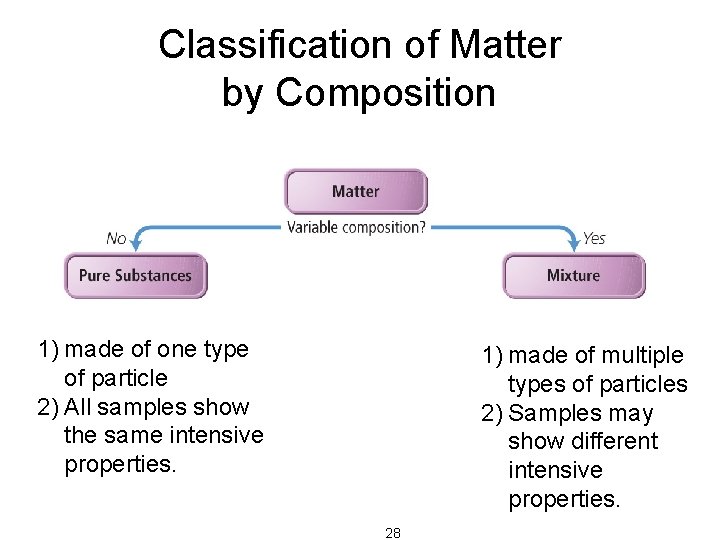
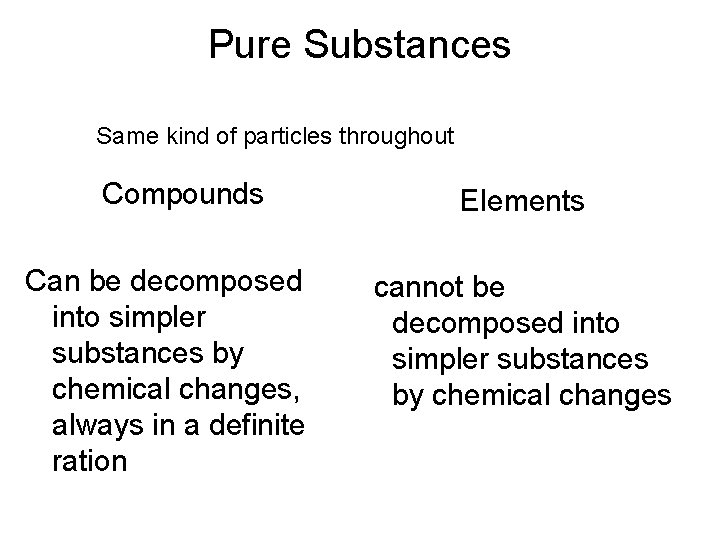
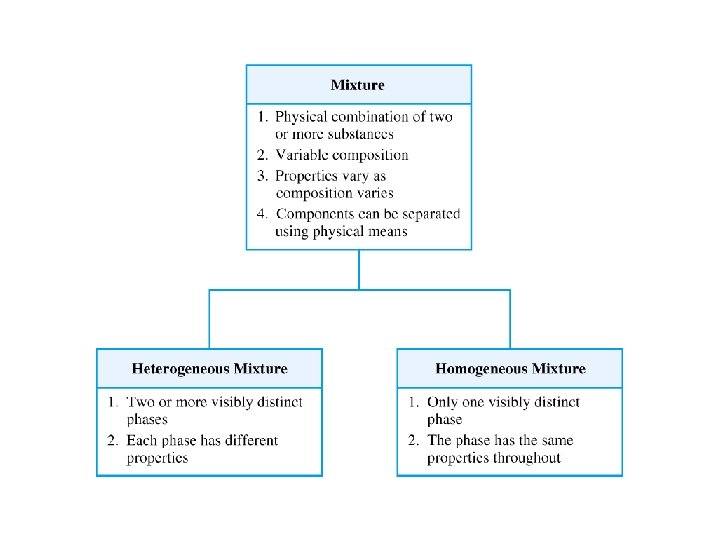
Classification of Matter by Composition • Matter whose composition does not change from one sample to another is called a pure substance. – made of a single type of atom or molecule – Because the composition of a pure substance is always the same, all samples have the same characteristics. • Matter whose composition may vary from one sample to another is called a mixture. – two or more types of atoms or molecules combined in variable proportions – Because composition varies, different samples have different characteristics. 27

Classification of Matter by Composition 1) made of one type of particle 2) All samples show the same intensive properties. 1) made of multiple types of particles 2) Samples may show different intensive properties. 28

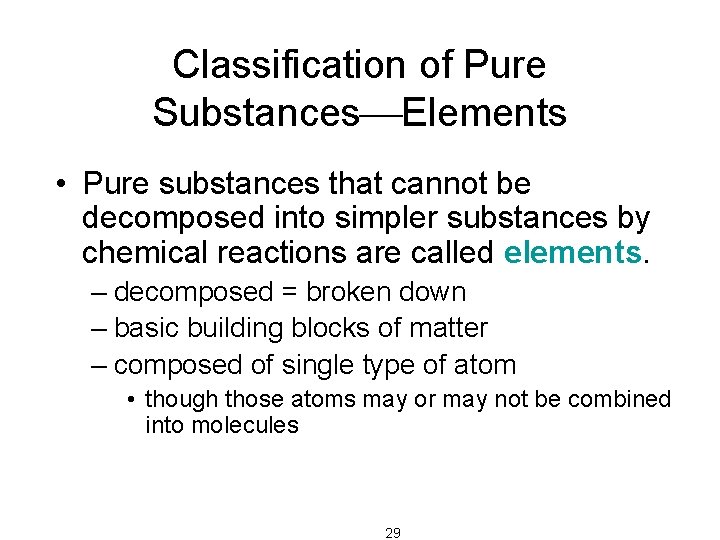
Classification of Pure Substances Elements • Pure substances that cannot be decomposed into simpler substances by chemical reactions are called elements. – decomposed = broken down – basic building blocks of matter – composed of single type of atom • though those atoms may or may not be combined into molecules 29

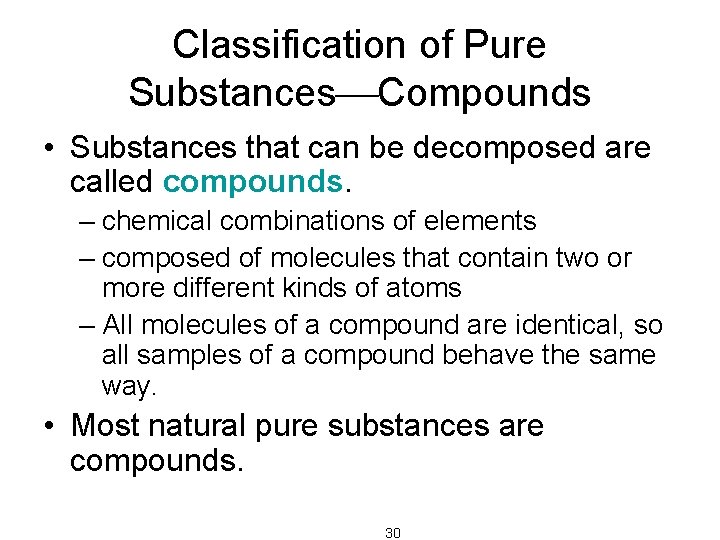
Classification of Pure Substances Compounds • Substances that can be decomposed are called compounds. – chemical combinations of elements – composed of molecules that contain two or more different kinds of atoms – All molecules of a compound are identical, so all samples of a compound behave the same way. • Most natural pure substances are compounds. 30

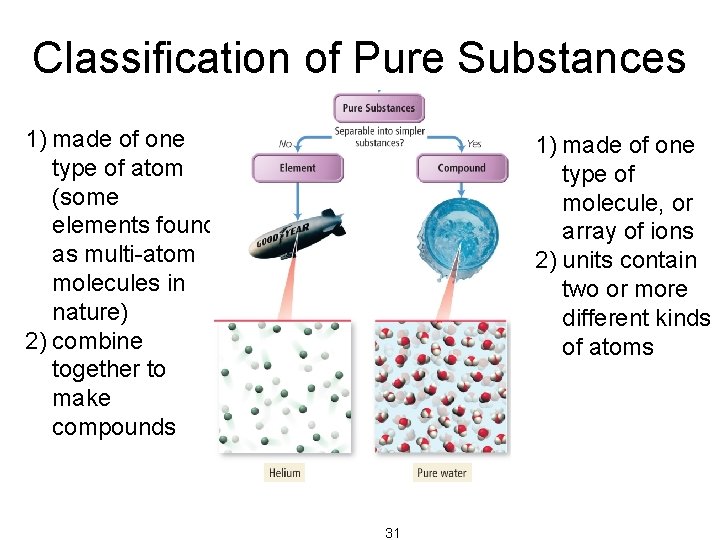
Classification of Pure Substances 1) made of one type of atom (some elements found as multi-atom molecules in nature) 2) combine together to make compounds 1) made of one type of molecule, or array of ions 2) units contain two or more different kinds of atoms 31

B. Pure Substances • Element – composed of identical atoms – EX: copper wire, aluminum foil

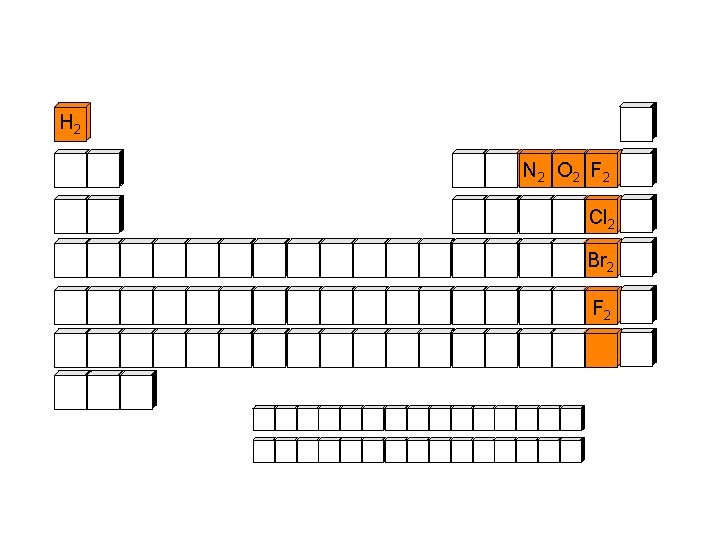
Define Ø Molecule – a combination of 2 0 r more atoms (same or different) that are covalently bonded. Ø A molecule is the smallest particle of a substance which exhibits the physical and chemical characteristics of the substance. Ø Diatomic molecules of elements : H 2 O 2 Cl 2 N 2 F 2 Br 2 I 2

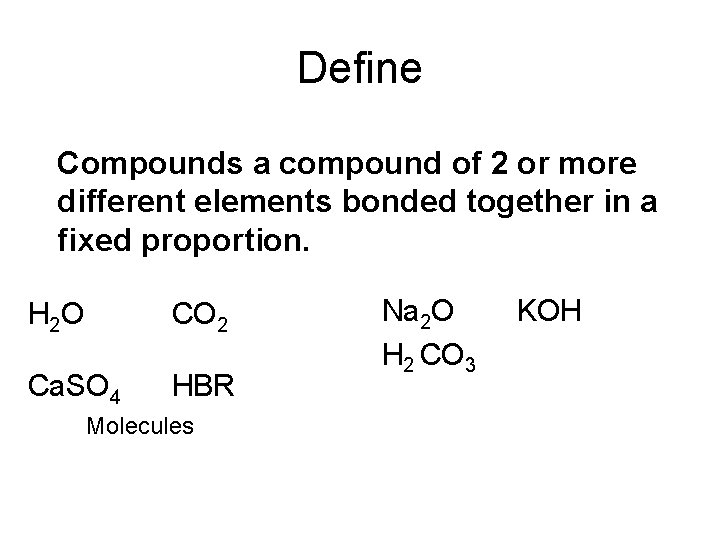
Define Compounds a compound of 2 or more different elements bonded together in a fixed proportion. H 2 O CO 2 Ca. SO 4 HBR Molecules Na 2 O H 2 CO 3 KOH

B. Pure Substances • Compound – composed of 2 or more elements in a fixed ratio – properties differ from those of individual elements – EX: table salt (Na. Cl)

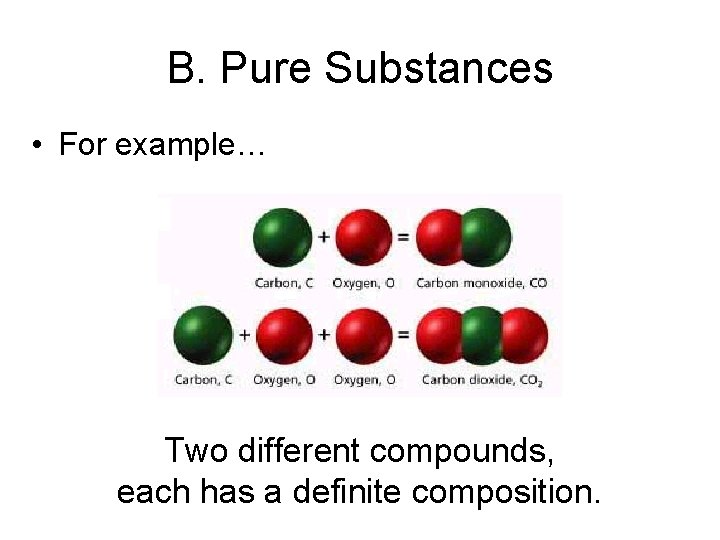
B. Pure Substances • For example… Two different compounds, each has a definite composition.

Compounds Slight differences in combinations of atoms can have large difference in properties H 2 O- water, H 2 O 2 – hydrogen peroxide C 2 H 6 O – ethanol, drinkable C 2 H 6 O 2 – ethylene glycol, poisonous

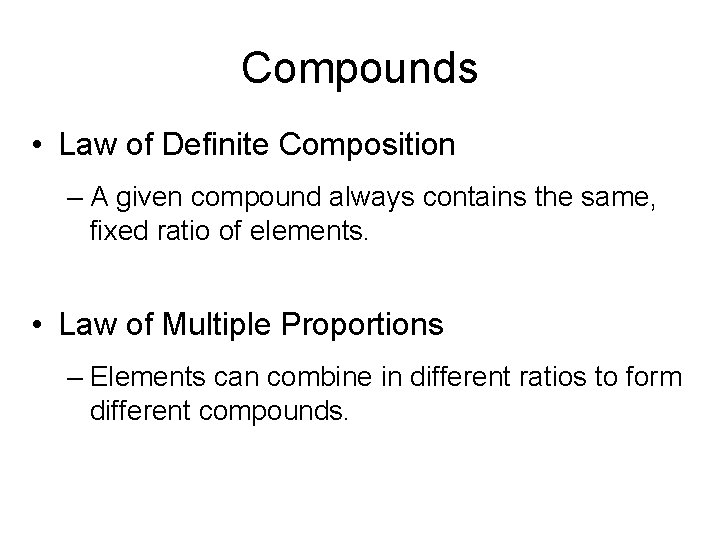
Compounds • Law of Definite Composition – A given compound always contains the same, fixed ratio of elements. • Law of Multiple Proportions – Elements can combine in different ratios to form different compounds.

Pure Substances Same kind of particles throughout Compounds Can be decomposed into simpler substances by chemical changes, always in a definite ration Elements cannot be decomposed into simpler substances by chemical changes

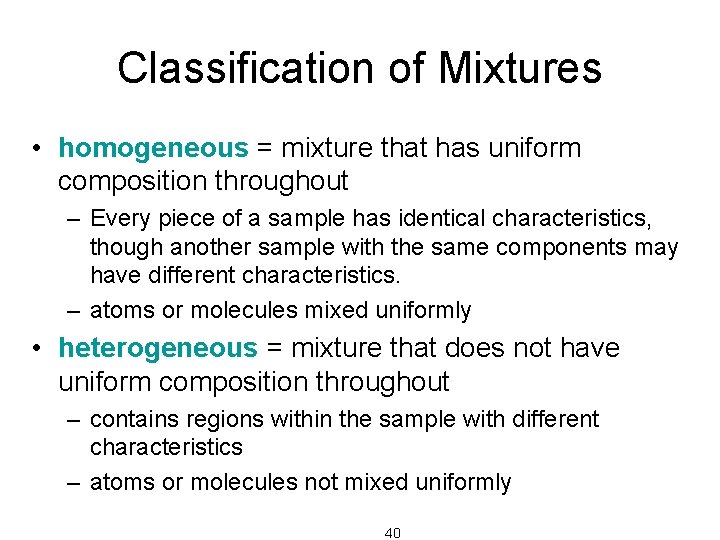
Classification of Mixtures • homogeneous = mixture that has uniform composition throughout – Every piece of a sample has identical characteristics, though another sample with the same components may have different characteristics. – atoms or molecules mixed uniformly • heterogeneous = mixture that does not have uniform composition throughout – contains regions within the sample with different characteristics – atoms or molecules not mixed uniformly 40

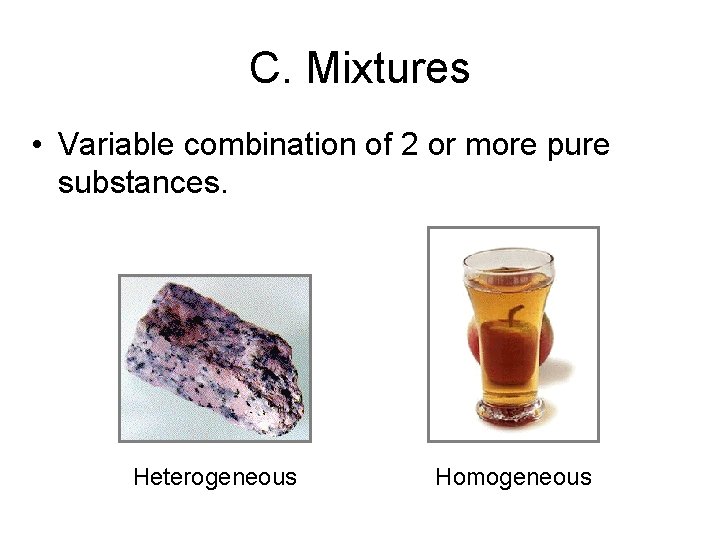
C. Mixtures • Variable combination of 2 or more pure substances. Heterogeneous Homogeneous

Classification of Mixtures 1) made of multiple substances, whose presence can be seen 2) Portions of a sample have different composition and properties. 1) made of multiple substances, but appears to be one substance 2) All portions of an individual sample have the same composition and properties. 42

Mixture • Mixtures are two or more substance that are not chemically combined. • Mixtures do not have a fixed composition • Mixtures do not have constant boiling points or melting points • Variable composition • Components retain their characteristic properties

Mixture • May be separated into pure substances by physical methods • Mixtures of different compositions may have widely different properties.

Types of mixtures ö Homogeneous mixture -1 phase -uniform properties in a sample -same composition in a sample eg: sugar and water Heterogeneous mixture -2 or more phases (with same or different physical states) -each phase has different properties eg: oil and water, sand water

C. Mixtures • Solution – homogeneous – very small particles – no Tyndall effect w particles don’t settle w EX: rubbing alcohol Tyndall Effect

C. Mixtures • Colloid – heterogeneous – medium-sized particles – Tyndall effect – particles don’t settle – EX: milk

C. Mixtures • Suspension – heterogeneous – large particles – Tyndall effect – particles settle – EX: fresh-squeezed lemonade

C. Mixtures • Examples: – mayonnaise – muddy water – fog – saltwater – Italian salad dressing colloid suspension colloid solution suspension

• Pure Substances and Mixtures

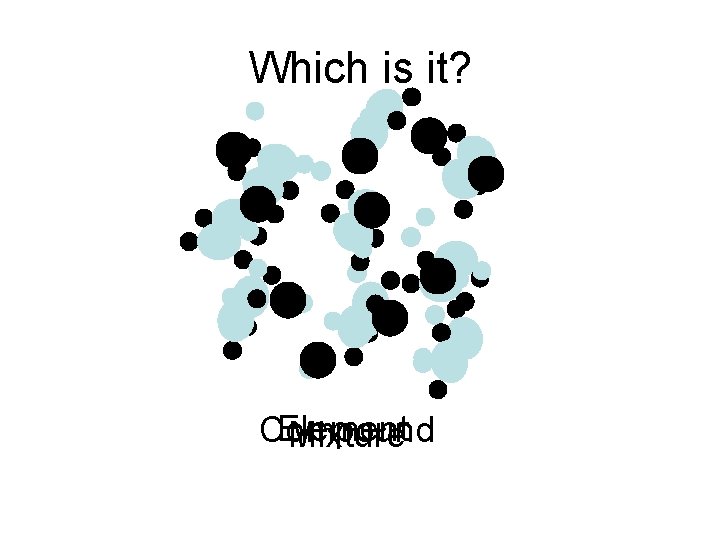
Which is it? Element Compound Mixture

Physical Separation Techniques ö By eye ö Filtration to separate solid and liquid ö Distillation to separate two or more liquids with different boiling points ö Chromatography to separate pure liquids or solutions of compounds



A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions no Element

PRACTICE PROBLEMS #4 1. Classify the following as an element, compound, or mixture (heterogeneous or homogeneous). E HO ö _____ air _____ oxygen ö _____ tin can _____ sugar E C HO HE ö _____ Windex _____ crude oil HO HE ö _____ suntan lotion _____ gummi bear 2. A white solid is dissolved in water. The resulting colorless, clear liquid is boiled in a beaker until dryness. White crystals remain in the beaker. The Homogeneous mixture liquid can be classified as a(n) _______. 3. Classify the following as physical or chemical changes. CC CC ö _____ photosynthesis _____ baking PC PC ö _____ writing with pencil _____ snowing

GROUP STUDY PROBLEM #4 1. Classify the following as an element, compound, or mixture (heterogeneous or homogeneous). ö _____ wine _____ root beer ö _____ penny _____ table salt ö _____ Bleach _____ wood ö _____ diamond _____ vinegar 2. A clear blue liquid in an open beaker was left in the hood. After 1 week, the beaker contained only blue crystals. The original liquid can be classified as a(n) _______. 3. Classify the following as physical or chemical changes. ö _____ perspiration _____ sugar dissolving ö _____ fermentation _____ aging

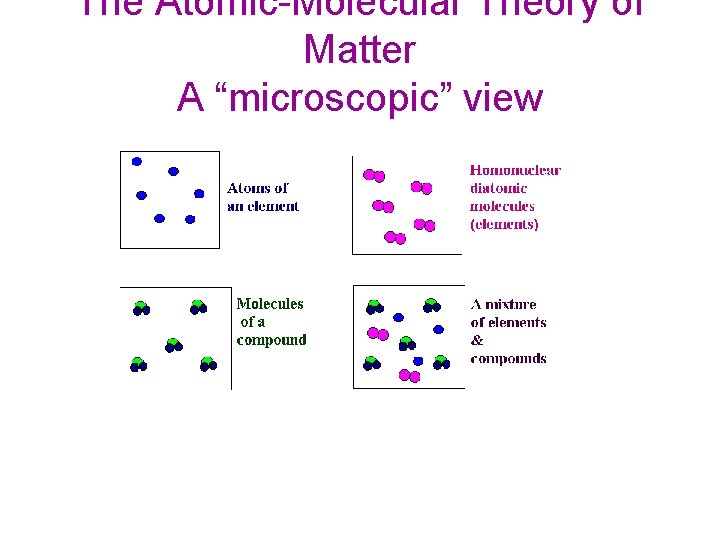
The Atomic-Molecular Theory of Matter A “microscopic” view

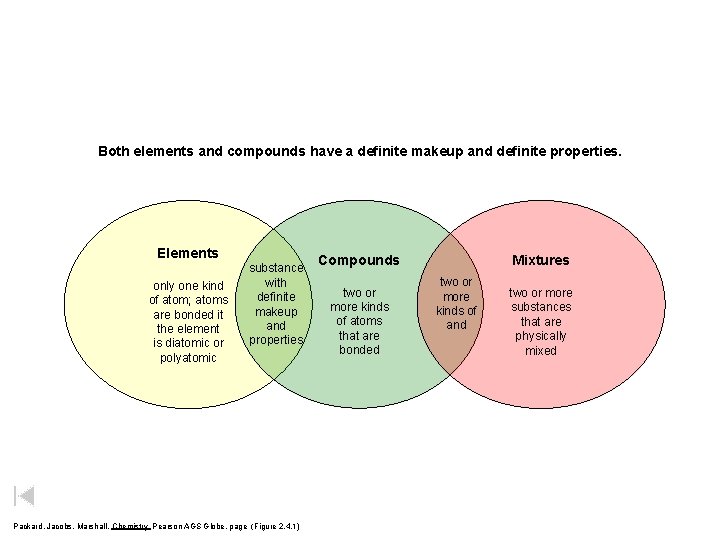
Both elements and compounds have a definite makeup and definite properties. Elements only one kind of atom; atoms are bonded it the element is diatomic or polyatomic substance with definite makeup and properties Packard, Jacobs, Marshall, Chemistry Pearson AGS Globe, page (Figure 2. 4. 1) Compounds two or more kinds of atoms that are bonded Mixtures two or more kinds of and two or more substances that are physically mixed

Changes in Matter • Changes that alter the state or appearance of the matter without altering the composition are called physical changes. • Changes that alter the composition of the matter are called chemical changes. – During the chemical change, the atoms that are present rearrange into new molecules, but all of the original atoms are still present. 60

What is a Phase Change? • Is a change from one state of matter (solid, liquid, gas) to another. • Phase changes are physical changes because: - It only affects physical appearance, not chemical make-up. - Reversible

Physical Changes in Matter The boiling of water is a physical change. The water molecules are separated from each other, but their structure and composition do not change. 62

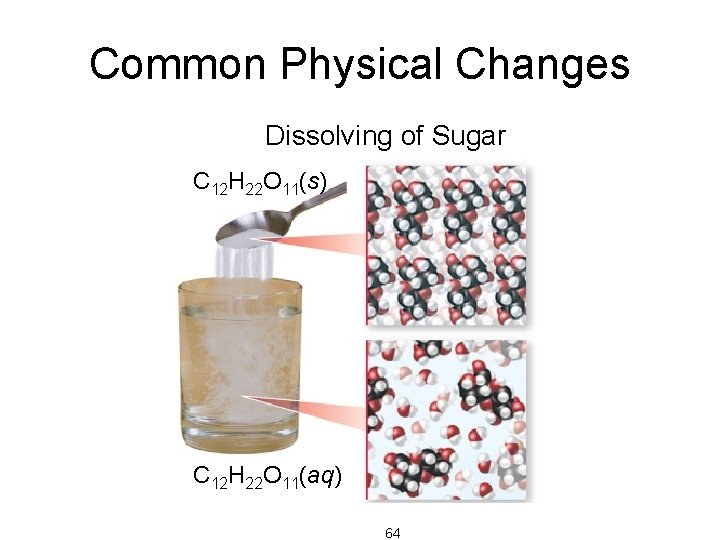
Common Physical Changes • processes that cause changes in the matter that do not change its composition • state changes Subliming of Dry Ice CO 2(g) Dry Ice – boiling/condensing – melting/freezing – subliming CO 2(s) • dissolving Dissolving of Sugar 63

Common Physical Changes Dissolving of Sugar C 12 H 22 O 11(s) C 12 H 22 O 11(aq) 64

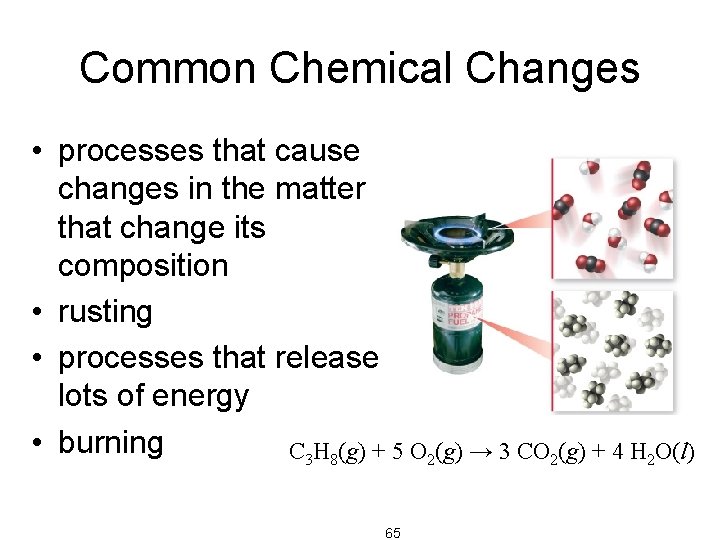
Common Chemical Changes • processes that cause changes in the matter that change its composition • rusting • processes that release lots of energy • burning C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) 65

What happens during a phase change? • During a phase change, heat energy is either absorbed or released. • Heat energy is released as molecules slow down and move closer together. • Heat energy is absorbed as molecules speed up and expand.

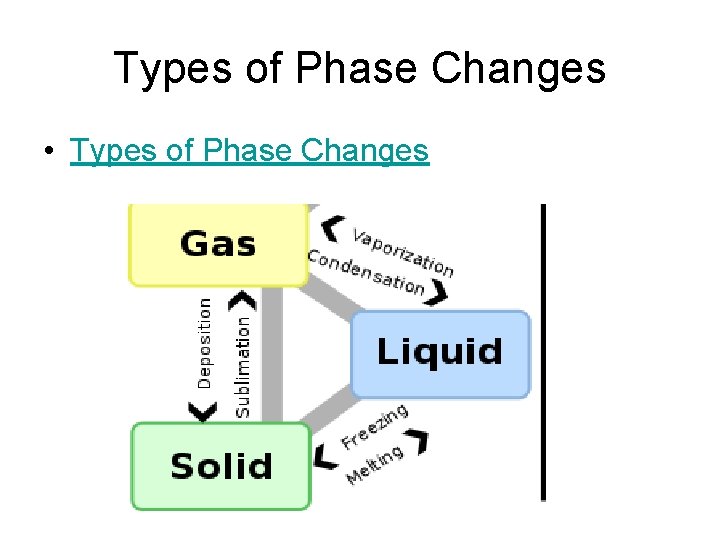
Types of Phase Changes • Types of Phase Changes

Melting • Phase change from a solid to a liquid • Molecules speed up, move farther apart, and absorb heat energy

PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

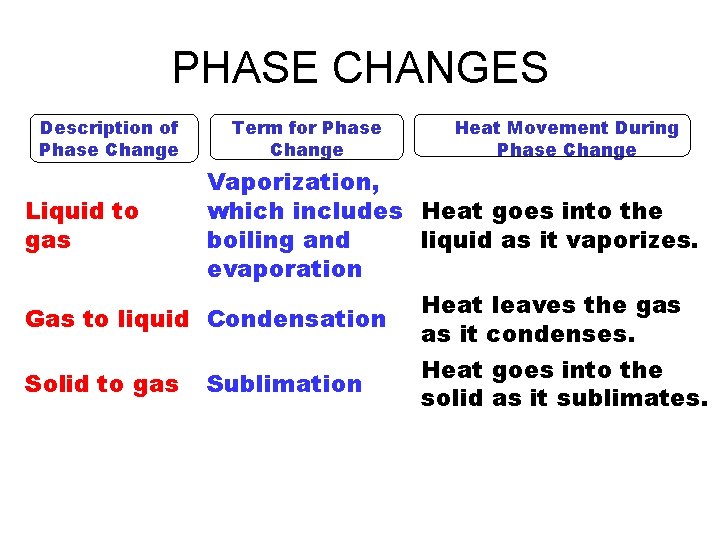
PHASE CHANGES Description of Phase Change Liquid to gas Term for Phase Change Vaporization, which includes Heat goes into the boiling and liquid as it vaporizes. evaporation Gas to liquid Condensation Solid to gas Heat Movement During Phase Change Sublimation Heat leaves the gas as it condenses. Heat goes into the solid as it sublimates.

Freezing • Phase Change from a liquid to a solid • Molecule slow down, move closer together and release heat energy.

Vaporization (Boiling) • Phase change from a liquid to gas. It occurs at the boiling point of matter. • Molecules speed up, move farther apart, and absorb heat energy.

Evaporation • Phase change from a liquid to a gas on the surface of a liquid (occurs at all temperatures). • Molecules speed up, move farther apart, and absorb heat energy.

Condensation • Phase change from a gas to a liquid. • Molecule slow down, move closer together and release heat energy.

Sublimation • Phase change from a solid to a gas. • Molecules speed up, move farther apart, and absorb heat energy.

Deposition • Phase change from a gas to a solid. • Molecules slow down, move closer together and release heat energy.

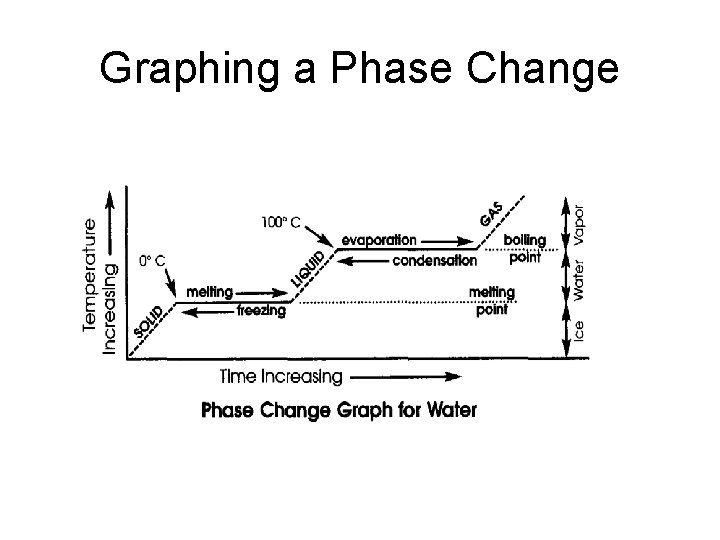
Graphing a Phase Change


Melting & Boiling Points • Melting Point: The temperature at which a solid changes into a liquid. • Boiling Point: The temperature at which a liquid changes into a gas. • What is a Freezing point? Compare the freezing and melting points of water.

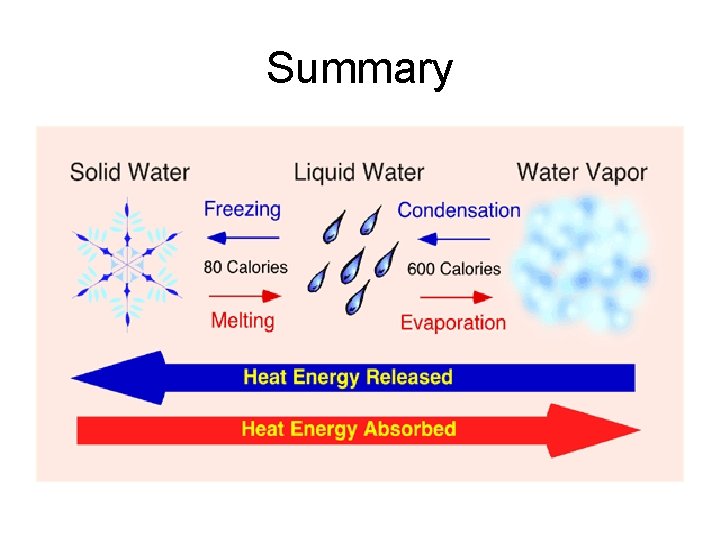
Summary

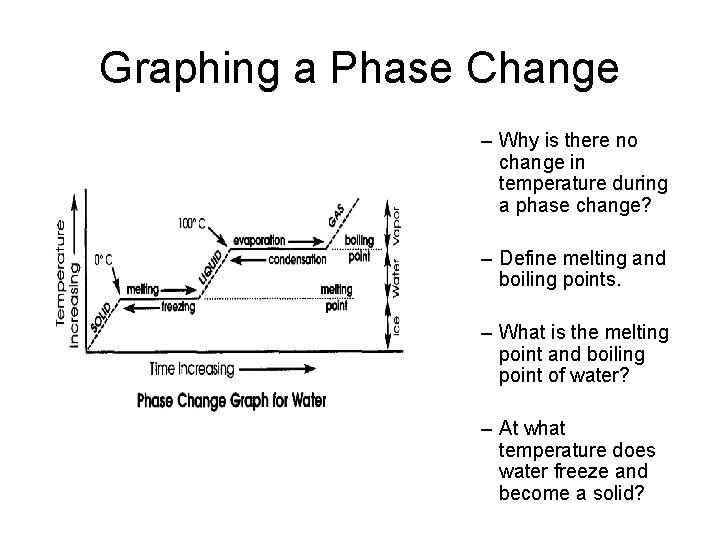
Graphing a Phase Change – Why is there no change in temperature during a phase change? – Define melting and boiling points. – What is the melting point and boiling point of water? – At what temperature does water freeze and become a solid?

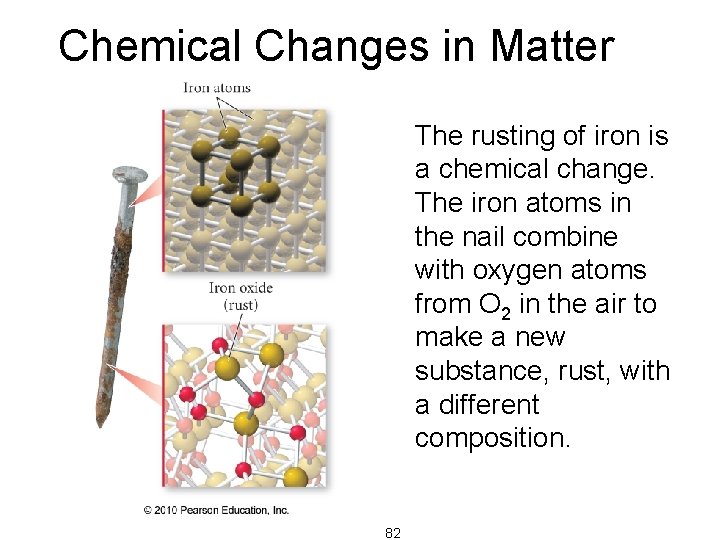
Chemical Changes in Matter The rusting of iron is a chemical change. The iron atoms in the nail combine with oxygen atoms from O 2 in the air to make a new substance, rust, with a different composition. 82

What is a Chemical Change? • When a substance undergoes a chemical change, it is changed into a different substance with different properties. Example: Baking a Cake

5 Signs of a Chemical Change 1. Color Change 2. Precipitation

5 Signs of a Chemical Change 3. Gas Production 4. Temperature Change

5 Signs of a Chemical Change 5. Changes in Characteristic Properties (odor, light given off)

Properties of Matter • Physical properties are the characteristics of matter that can be changed without changing its composition. – characteristics that are directly observable • Chemical properties are the characteristics that determine how the composition of matter changes as a result of contact with other matter or the influence of energy. – characteristics that describe the behavior of matter 87

1. A. Physical Change B. Chemical Change Rusting nails

2. A. Physical Change B. Chemical Change Effervescent tablet

3. A. Physical Change B. Chemical Change Cut paper

4. A. Physical Change B. Chemical Change Vinegar and Baking soda

5. A. Physical Change B. Chemical Change Salt and water

6. A. Physical Change B. Chemical Change Broken glass

7. A. Physical Change B. Chemical Change Burning wood

8. A. Physical Change B. Chemical Change Ice melting

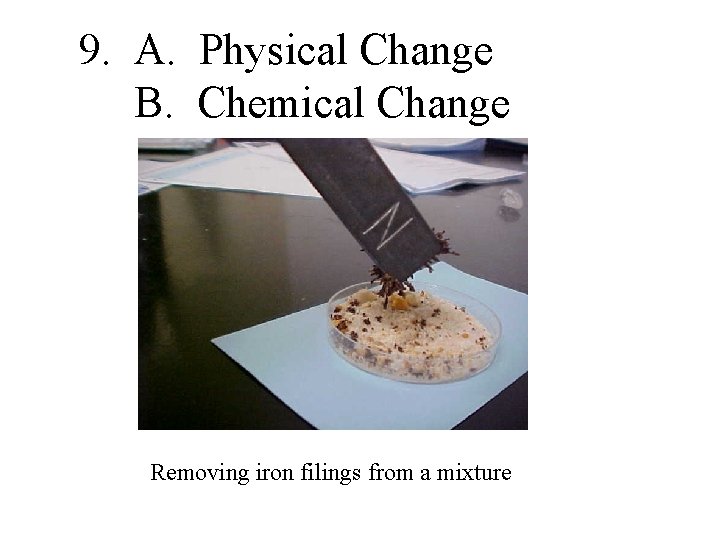
9. A. Physical Change B. Chemical Change Removing iron filings from a mixture

10. A. Physical Change B. Chemical Change Boiling water

Mass • A measure of how much matter is in an object.

Weight • A measure of the force of gravity on an object.

Volume • The amount of space that matter occupies.

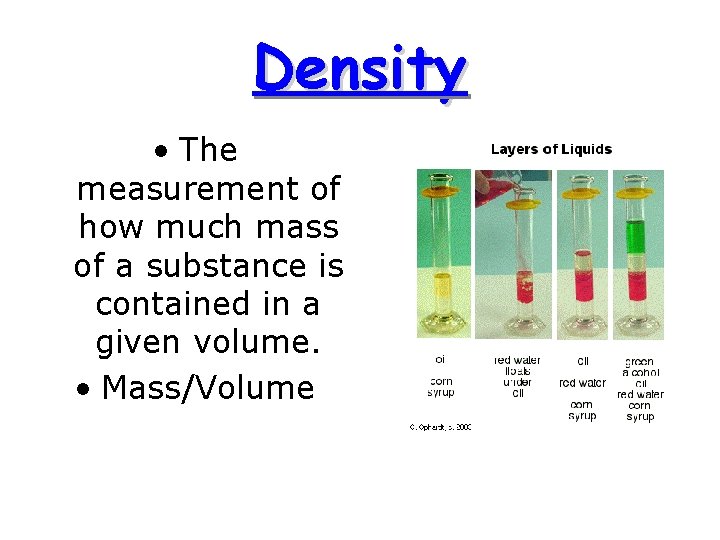
Density • The measurement of how much mass of a substance is contained in a given volume. • Mass/Volume

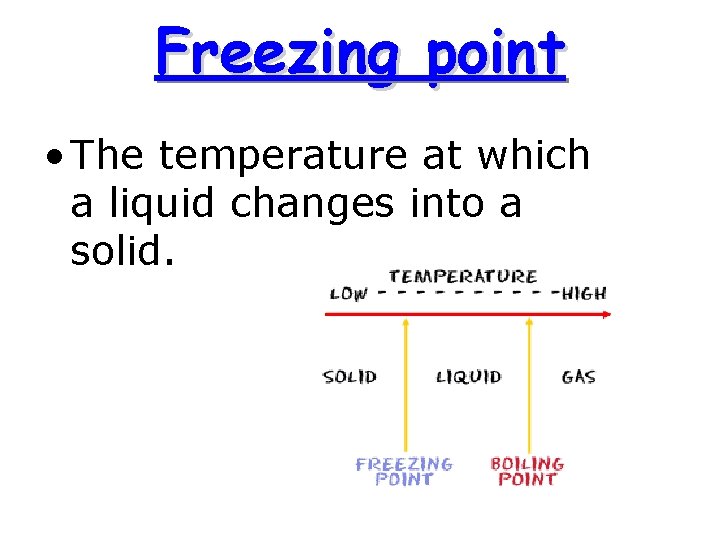
Freezing point • The temperature at which a liquid changes into a solid.

Boiling point • The boiling point of an element or compound means the temperature at which the liquid form of an element or compound is at equilibrium with the gaseous form. • the boiling point of water is 100 degrees Celsius.

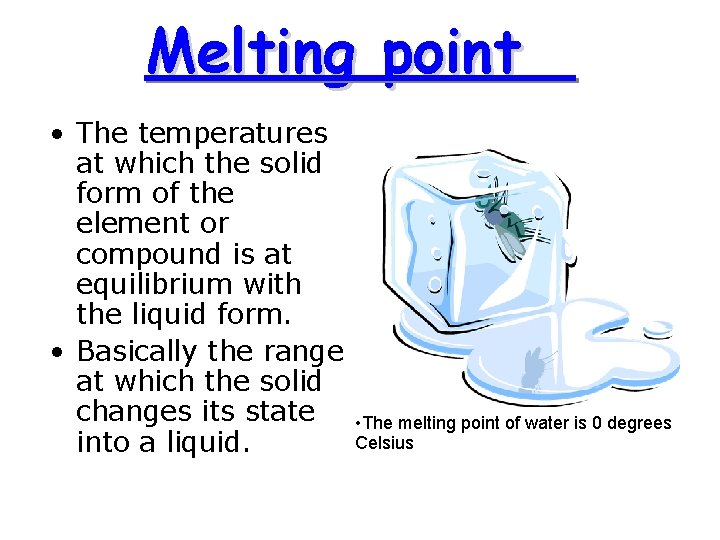
Melting point • The temperatures at which the solid form of the element or compound is at equilibrium with the liquid form. • Basically the range at which the solid changes its state into a liquid. • The melting point of water is 0 degrees Celsius

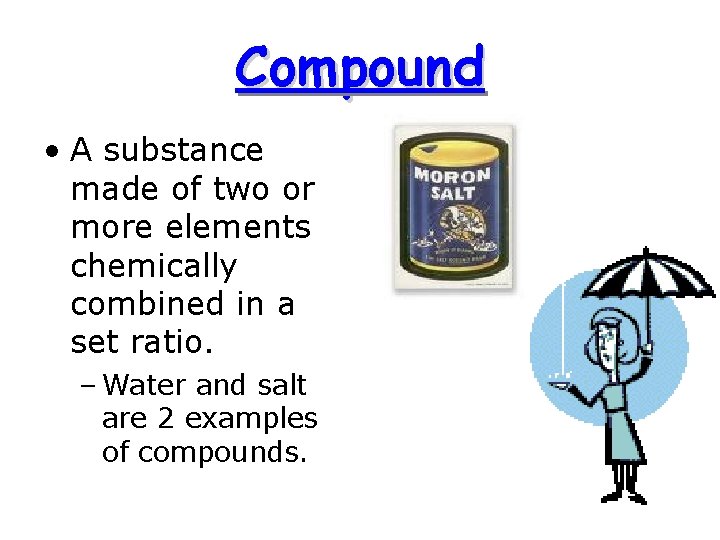
Compound • A substance made of two or more elements chemically combined in a set ratio. – Water and salt are 2 examples of compounds.

All substances have properties… Including people! Example: People can be identified by their … Face (shape, Voice Height Finger prints Teeth DNA expressions) Eye color Hair color

What are properties? • Matter has observable and measurable qualities. • We can use general properties to identify substances. • Two basic types of properties of matter: Physical properties and Chemical properties:

Physical Properties • Physical properties are used to identify, describe and classify matter. – Characteristic of a substance that can be observed (using your senses) without changing the substance into something else. Hardness Texture Color Odor Taste Temperature

More EXAMPLES Physical • size, shape, freezing point, boiling point, melting point, magnetism, viscosity, density, luster and many more. – Viscosity - The resistance of a liquid to flowing. – Examples: – Low viscosity-water, rubbing alcohol – High viscosity-honey

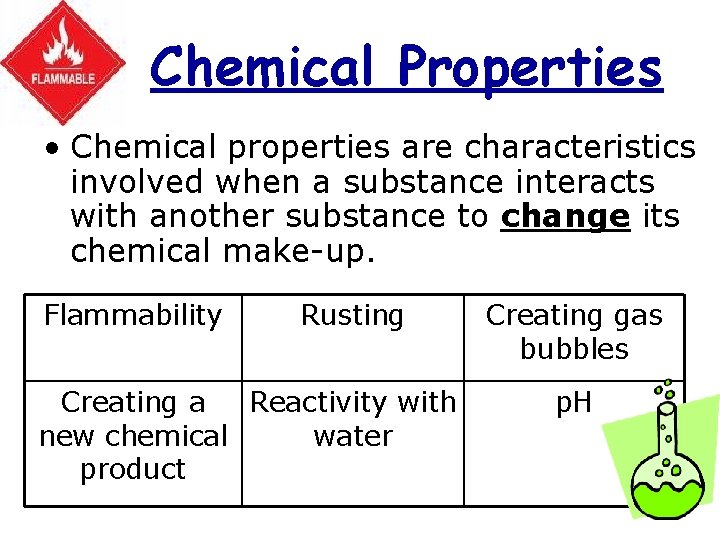
Chemical Properties • Chemical properties are characteristics involved when a substance interacts with another substance to change its chemical make-up. Flammability Rusting Creating a Reactivity with new chemical water product Creating gas bubbles p. H

Alike? Different? • Draw a double bubble map in your notes to compare and contrast physical and chemical properties.

Diatomic Elements, 1 and 7 H 2 N 2 O 2 F 2 Cl 2 Br 2 F 2

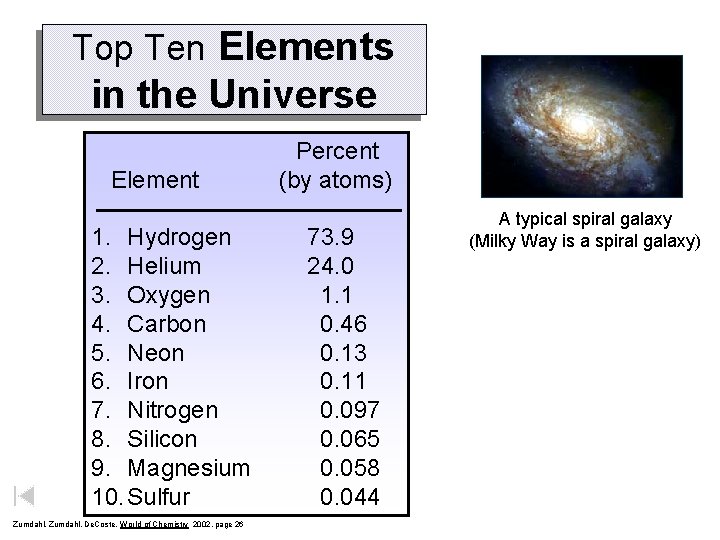
Top Ten Elements in the Universe Element 1. Hydrogen 2. Helium 3. Oxygen 4. Carbon 5. Neon 6. Iron 7. Nitrogen 8. Silicon 9. Magnesium 10. Sulfur Zumdahl, De. Coste, World of Chemistry 2002, page 26 Percent (by atoms) 73. 9 24. 0 1. 1 0. 46 0. 13 0. 11 0. 097 0. 065 0. 058 0. 044 A typical spiral galaxy (Milky Way is a spiral galaxy)

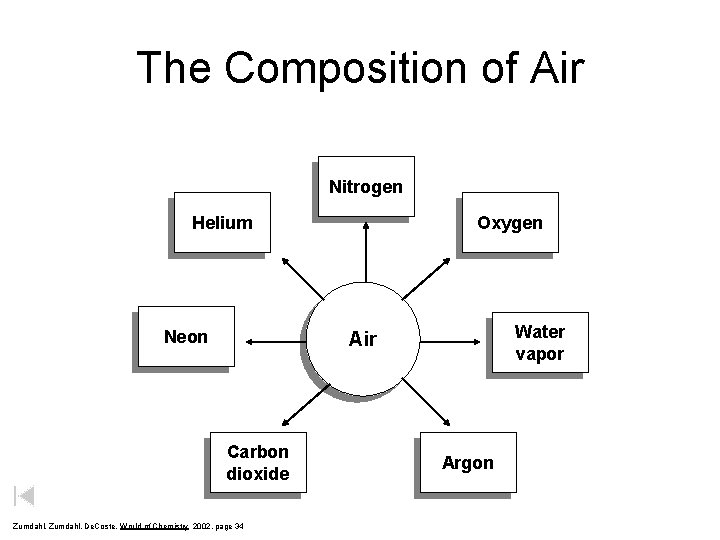
The Composition of Air Nitrogen Helium Neon Oxygen Water vapor Air Carbon dioxide Zumdahl, De. Coste, World of Chemistry 2002, page 34 Argon

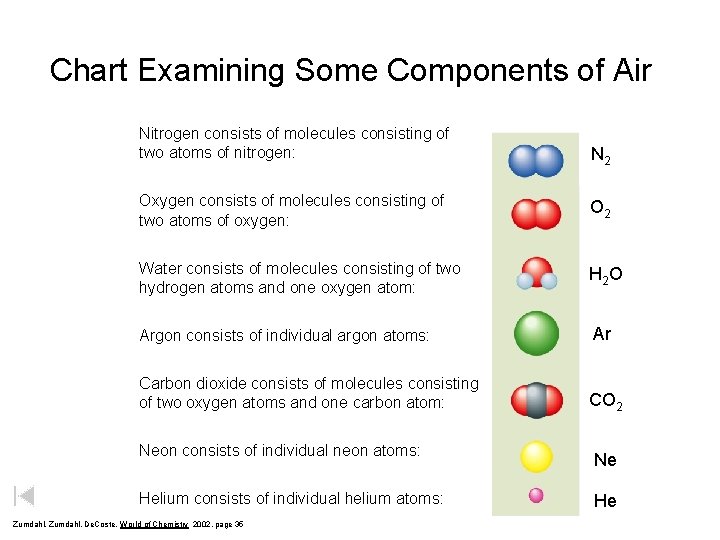
Chart Examining Some Components of Air Nitrogen consists of molecules consisting of two atoms of nitrogen: N 2 Oxygen consists of molecules consisting of two atoms of oxygen: O 2 Water consists of molecules consisting of two hydrogen atoms and one oxygen atom: H 2 O Argon consists of individual argon atoms: Ar Carbon dioxide consists of molecules consisting of two oxygen atoms and one carbon atom: CO 2 Neon consists of individual neon atoms: Helium consists of individual helium atoms: Zumdahl, De. Coste, World of Chemistry 2002, page 35 Ne He
 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Plasma particles arrangement
Plasma particles arrangement Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Slave states free states
Slave states free states Southern states of america
Southern states of america Big states vs small states guard against tyranny
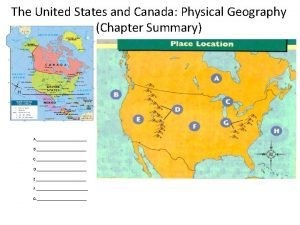
Big states vs small states guard against tyranny United states and canada physical map
United states and canada physical map The physical geography of the united states and canada
The physical geography of the united states and canada Function of grey matter and white matter
Function of grey matter and white matter Optic tract
Optic tract Gray matter and white matter
Gray matter and white matter What is grey matter
What is grey matter States of matter foldable
States of matter foldable Four states of matter
Four states of matter Four states of matter
Four states of matter States of matter
States of matter Which state of matter has the most thermal energy
Which state of matter has the most thermal energy Changing state
Changing state Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter State of matter venn diagram
State of matter venn diagram The kinetic theory of matter states that
The kinetic theory of matter states that Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 States of matter basics
States of matter basics States of matter foldable
States of matter foldable What is heat energy?
What is heat energy? Uses of heat
Uses of heat The fundamental difference between states of matter is the
The fundamental difference between states of matter is the States of matter graph
States of matter graph Phase change concept map
Phase change concept map Concept map of matter solid liquid and gas
Concept map of matter solid liquid and gas Whats the four states of matter
Whats the four states of matter States of matter
States of matter States of matter jeopardy
States of matter jeopardy Chemical equation with states of matter
Chemical equation with states of matter States of matter
States of matter 4 phases of matter
4 phases of matter States of matter solid liquid gas
States of matter solid liquid gas Interconversion of states of matter
Interconversion of states of matter Matter flow chart chemistry
Matter flow chart chemistry Five states of matter
Five states of matter States of matter objectives
States of matter objectives States of matter
States of matter 4 fe
4 fe Solid matters
Solid matters States of matter jeopardy
States of matter jeopardy 5 states of matter
5 states of matter Jeopardy states of matter
Jeopardy states of matter Physical behavior of matter heating and cooling curves
Physical behavior of matter heating and cooling curves Physical behavior of matter heating and cooling curves
Physical behavior of matter heating and cooling curves Eating food physical or chemical change
Eating food physical or chemical change Physical geography usa
Physical geography usa Physical geography of the united states
Physical geography of the united states What states are west of the mississippi river
What states are west of the mississippi river Physical states
Physical states Broken stream fire nozzle
Broken stream fire nozzle Non physical fences example
Non physical fences example What is physical fitness test in mapeh
What is physical fitness test in mapeh Classification and properties of matter
Classification and properties of matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Ecological succession
Ecological succession Changes of matter examples
Changes of matter examples Physical state of matter
Physical state of matter Topic 4 physical behavior of matter
Topic 4 physical behavior of matter Physical behavior of matter
Physical behavior of matter Physical behavior of matter
Physical behavior of matter Physical properties of matter jeopardy
Physical properties of matter jeopardy Matter graphic organizer
Matter graphic organizer Physical properties of matter
Physical properties of matter Chemical change meaning
Chemical change meaning Characteristics of tabulation
Characteristics of tabulation Classification of matter worksheet
Classification of matter worksheet What is classification of matter
What is classification of matter Classification of matter quiz
Classification of matter quiz Matter flowchart
Matter flowchart Classification of matter
Classification of matter Matter classification
Matter classification Pizza classification of matter
Pizza classification of matter Granite classification of matter
Granite classification of matter Properties of matter grade 7
Properties of matter grade 7 Classification of matter concept map
Classification of matter concept map Classification of matter
Classification of matter Matterlab
Matterlab Classification of matter
Classification of matter Types of colloids and examples
Types of colloids and examples Classification of matter
Classification of matter Homogeneous mixture
Homogeneous mixture Classification of matter lab
Classification of matter lab What is a heterogeneous mixture that never settles
What is a heterogeneous mixture that never settles Chapter 2 types of evidence
Chapter 2 types of evidence Eager learning algorithm
Eager learning algorithm Traditional classification vs modern classification
Traditional classification vs modern classification Northeast region states and capitals and abbreviations
Northeast region states and capitals and abbreviations Southeast states and capitals
Southeast states and capitals Virtual time and global states of distributed systems
Virtual time and global states of distributed systems The southwest states and capitals
The southwest states and capitals Awake united states!
Awake united states! Examples of elongated states
Examples of elongated states Map of the southwest region
Map of the southwest region Map of the world with latitude and longitude lines
Map of the world with latitude and longitude lines Lesson 2 kingdoms and states of africa
Lesson 2 kingdoms and states of africa Unit 2 the united states and canada
Unit 2 the united states and canada North and south states civil war
North and south states civil war Revolutions and national states in the atlantic world
Revolutions and national states in the atlantic world Prorupted state ap human geography
Prorupted state ap human geography Lesson quiz 13-1 kingdoms and states of medieval africa
Lesson quiz 13-1 kingdoms and states of medieval africa North and south states civil war
North and south states civil war Mali spice chart
Mali spice chart Bergonie and tribondeau law
Bergonie and tribondeau law Present simple fact examples
Present simple fact examples Chapter 13 kingdoms and states of medieval africa
Chapter 13 kingdoms and states of medieval africa Mandate of heaven dynasty
Mandate of heaven dynasty She's all states and all princes i
She's all states and all princes i