Classification of Matter Pure Substances Elements Compounds Mixtures
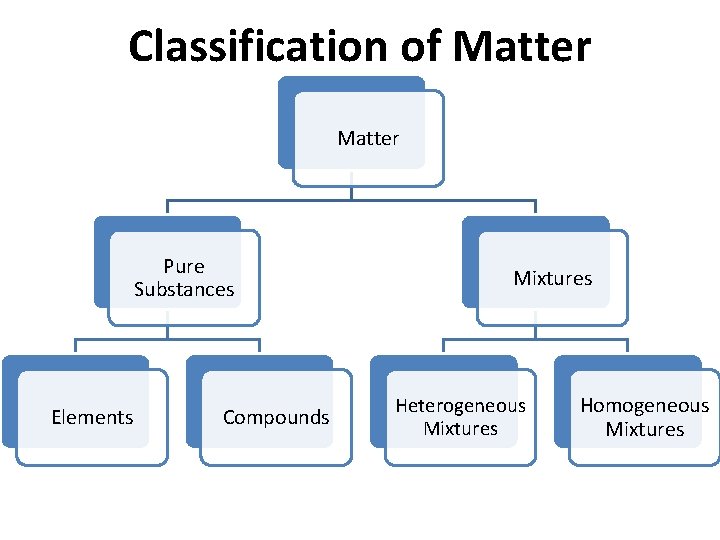
Classification of Matter Pure Substances Elements Compounds Mixtures Heterogeneous Mixtures Homogeneous Mixtures
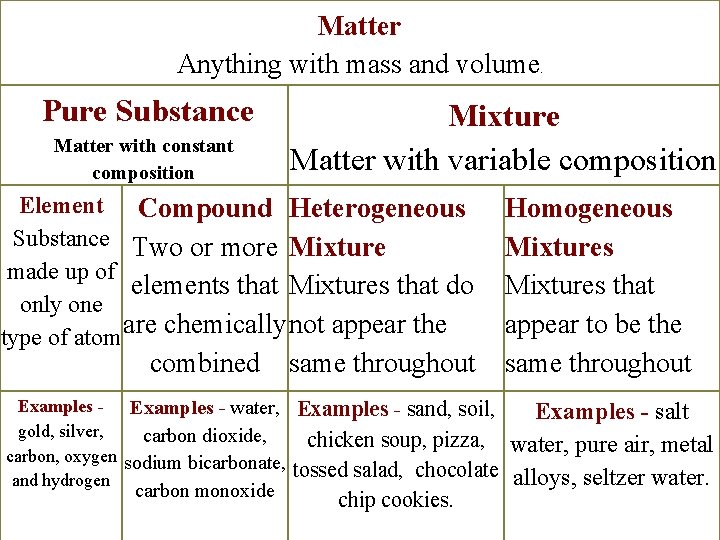
Matter Anything with mass and volume. Pure Substance Matter with constant composition Mixture Matter with variable composition Element Compound Heterogeneous Substance Two or more Mixture made up of elements that Mixtures that do only one are chemically not appear the type of atom combined Examples - water, gold, silver, carbon dioxide, carbon, oxygen sodium bicarbonate, and hydrogen same throughout Homogeneous Mixtures that appear to be the same throughout Examples - sand, soil, Examples - salt chicken soup, pizza, water, pure air, metal tossed salad, chocolate alloys, seltzer water. carbon monoxide chip cookies.
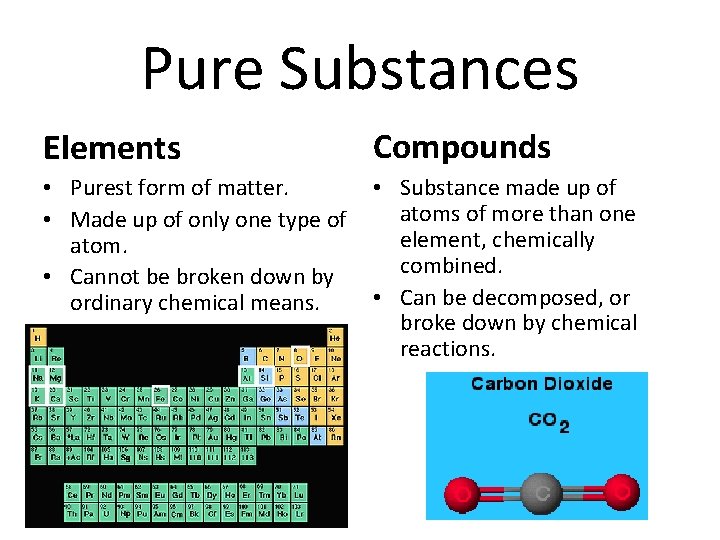
Pure Substances Elements Compounds • Purest form of matter. • Made up of only one type of atom. • Cannot be broken down by ordinary chemical means. • Substance made up of atoms of more than one element, chemically combined. • Can be decomposed, or broke down by chemical reactions.

What is the purest form of matter? A. B. C. D. Elements Compounds Mixtures Solutions
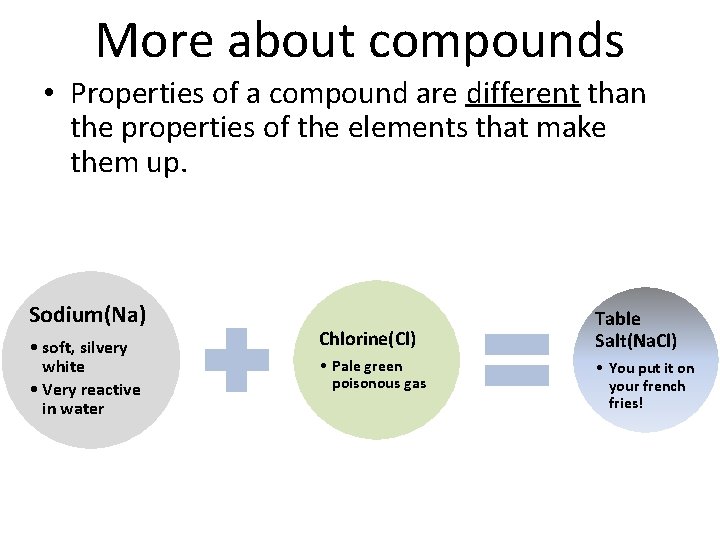
More about compounds • Properties of a compound are different than the properties of the elements that make them up. Sodium(Na) • soft, silvery white • Very reactive in water Chlorine(Cl) • Pale green poisonous gas Table Salt(Na. Cl) • You put it on your french fries!

Properties of Mixtures • • Each substance has its own unique properties Substances may change physical appearance. Substances can be present in any amount. Mixtures can be separated by ordinary means.
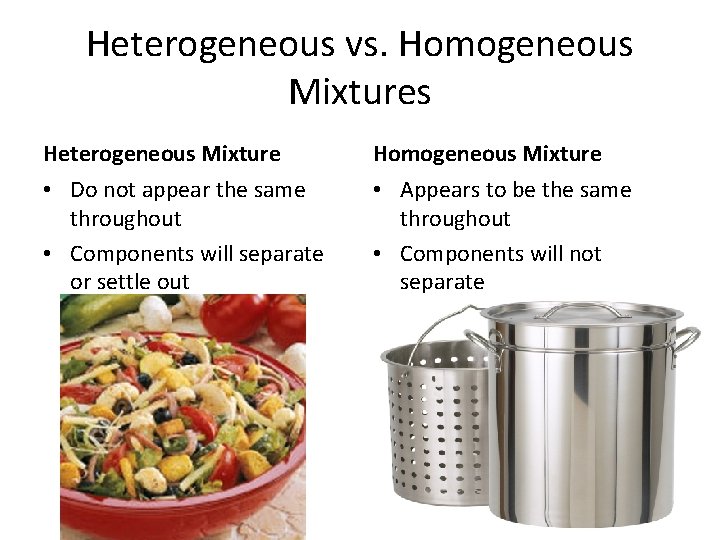
Heterogeneous vs. Homogeneous Mixtures Heterogeneous Mixture Homogeneous Mixture • Do not appear the same throughout • Components will separate or settle out • Appears to be the same throughout • Components will not separate


Suspensions • Type of mixture that will “settle” out!! • Will not scatter light(Can’t see a beam of light through it!!)!.

Colloids • Appear homogeneous to the naked eye. • Will scatter light(Can see a beam of light through it).

Solution • Type of homogeneous mixture in which one substance completely dissolves in another. • “Best mixed mixture!!” • 2 parts: 1. Solute—substance that dissolves 2. Solvent—substance that the solute dissolves in

Soluble vs. Insoluble Soluble Insoluble • A substance is said to be soluble in a substance, when it will dissolve in that substance. • A substance is said to be insoluble in a substance, when it will not dissolve in that substance. Some substances are soluble in one solvent, but insoluble in another solvent.

Solubility • Amount of solute that can be completely dissolved in a given amount of solvent. • Solubility Curve—Curve showing how temperature affects the solubility of a substance.

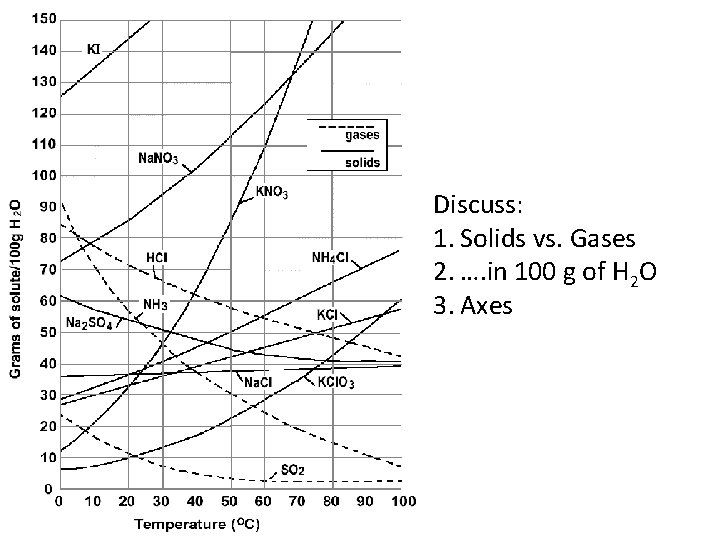
Discuss: 1. Solids vs. Gases 2. …. in 100 g of H 2 O 3. Axes
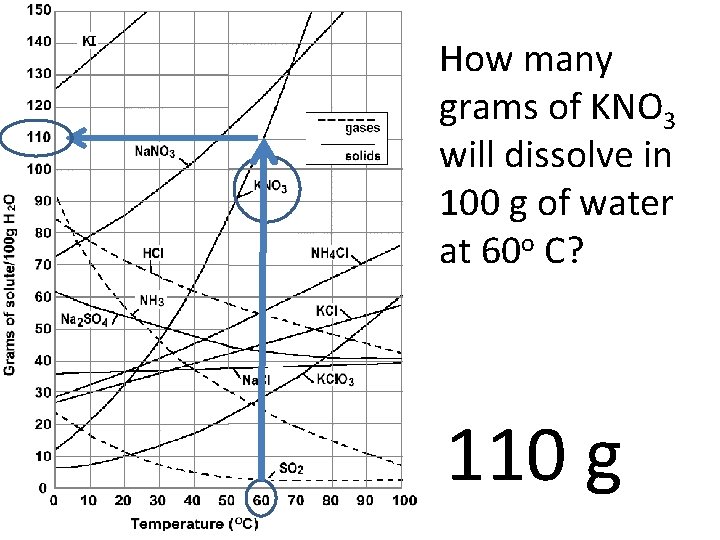
How many grams of KNO 3 will dissolve in 100 g of water at 60 o C? 110 g
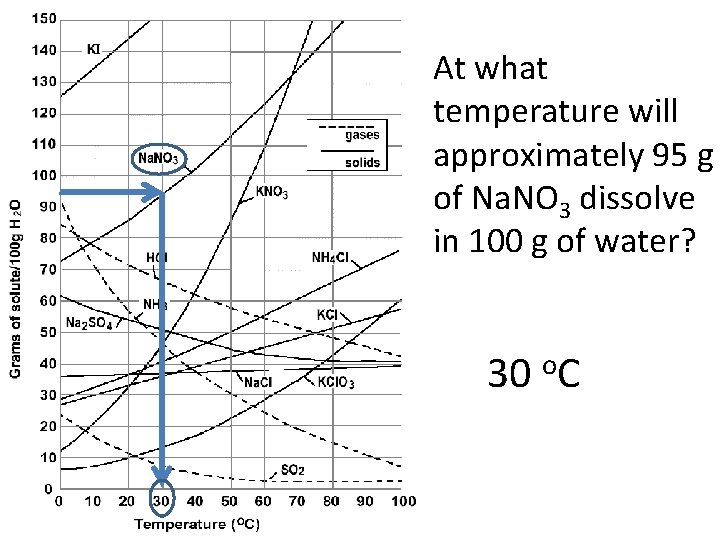
At what temperature will approximately 95 g of Na. NO 3 dissolve in 100 g of water? 30 o. C
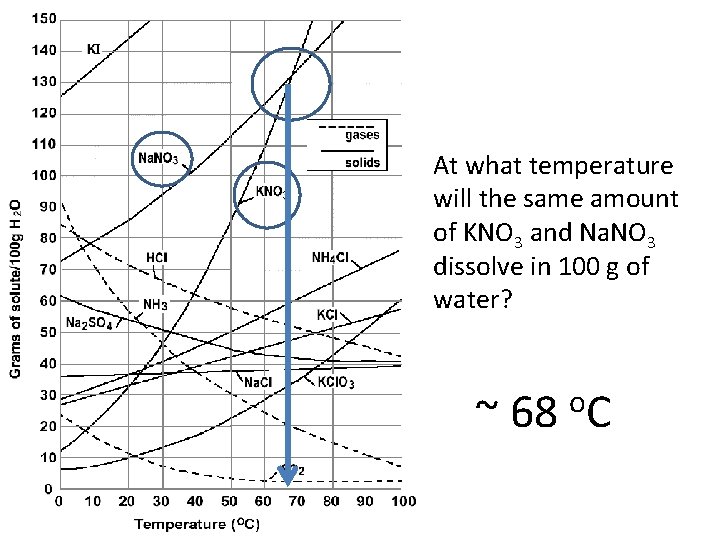
At what temperature will the same amount of KNO 3 and Na. NO 3 dissolve in 100 g of water? ~ 68 o. C
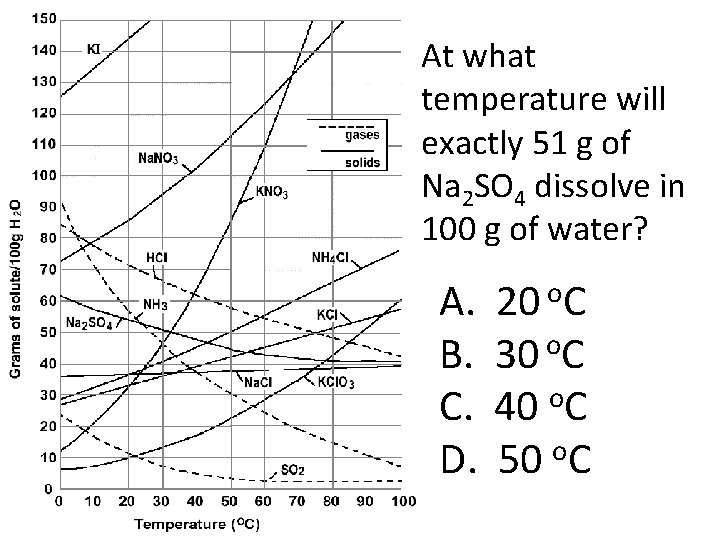
At what temperature will exactly 51 g of Na 2 SO 4 dissolve in 100 g of water? A. B. C. D. 20 o. C 30 o. C o 40 C o 50 C
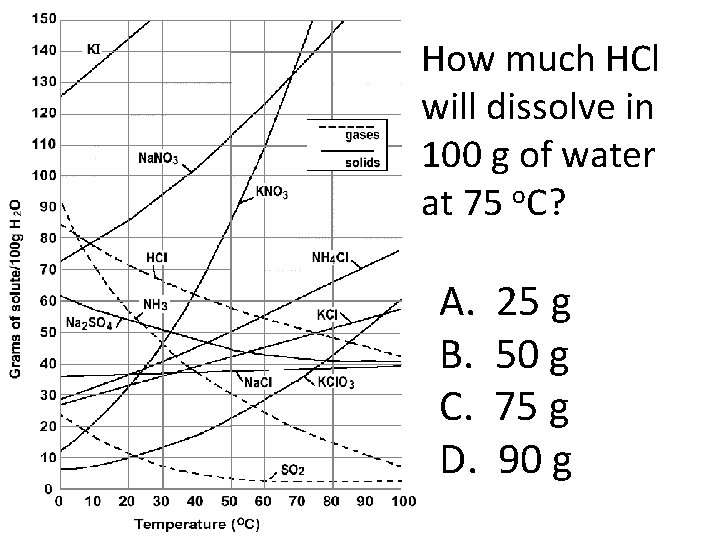
How much HCl will dissolve in 100 g of water at 75 o. C? A. B. C. D. 25 g 50 g 75 g 90 g
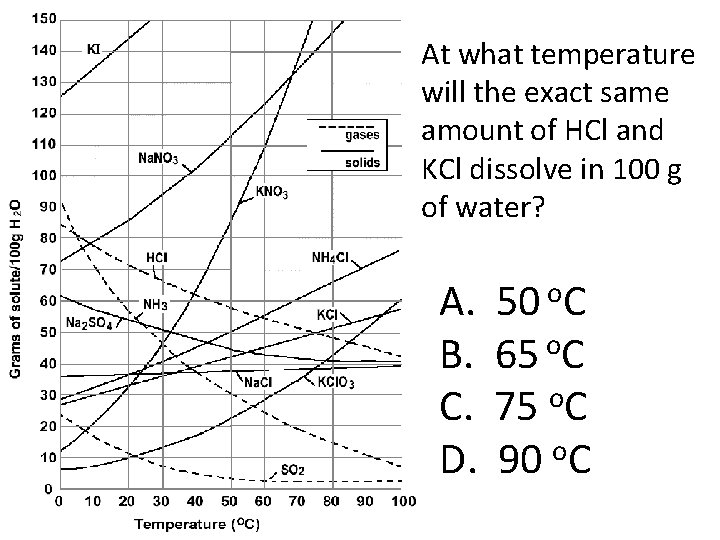
At what temperature will the exact same amount of HCl and KCl dissolve in 100 g of water? A. B. C. D. 50 o. C 65 o. C o 75 C o 90 C

Levels of Saturation 1. Unsaturated solution—solution “could” dissolve more solute. 2. Saturated solution—Solution has dissolved all the solute possible. 3. Supersaturated solution—Solution has dissolved all solute possible, but more solute is added anyway.
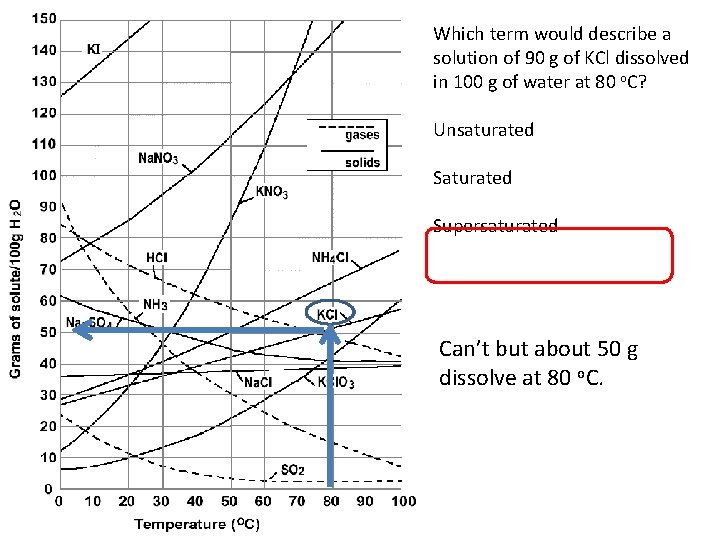
Which term would describe a solution of 90 g of KCl dissolved in 100 g of water at 80 o. C? Unsaturated Supersaturated Can’t but about 50 g dissolve at 80 o. C.
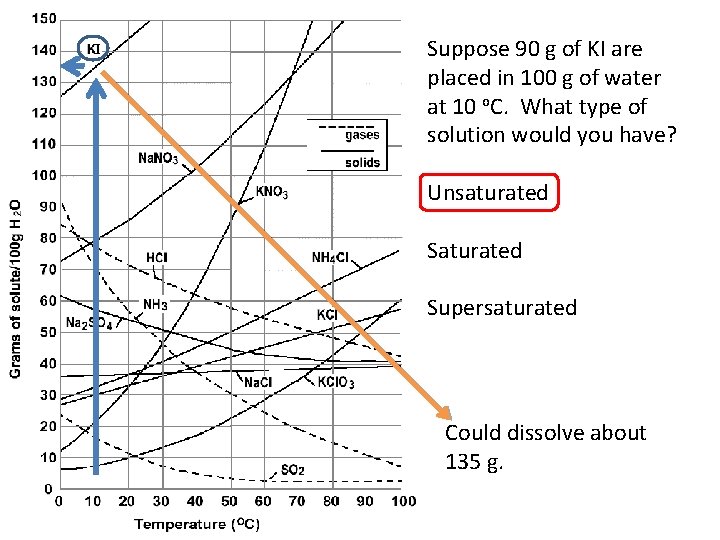
Suppose 90 g of KI are placed in 100 g of water at 10 o. C. What type of solution would you have? Unsaturated Supersaturated Could dissolve about 135 g.
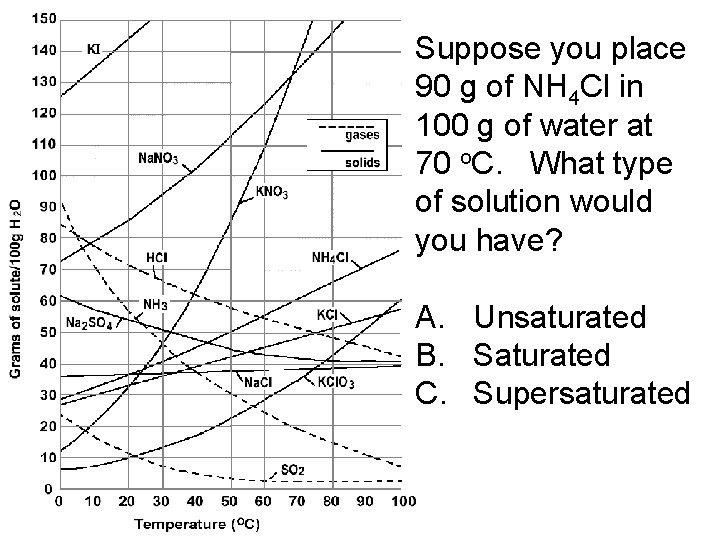
Suppose you place 90 g of NH 4 Cl in 100 g of water at 70 o. C. What type of solution would you have? A. Unsaturated B. Saturated C. Supersaturated
- Slides: 25