Classification of Matter Matter Everything is made up













- Slides: 16

Classification of Matter

Matter ö Everything is made up of tiny particles called atoms. ö We organize matter by how the atoms are connected (bonded)

2 major classifications categories ö 1. Pure Substance – made up of 1 kind of matter, from the periodic table ö 2. Mixture – made of 2 or more substances that are not chemically combined

1. Pure Substances A. Element w All matter composed of identical atoms, from the periodic table w EX: copper

A. Element 1. Monoatomic – 1 atom - EX: He 2. Diatomic – 2 atoms - EX: O 2 3. Polyatomic – 3 or more atoms - Ex: Al 3

B. Compound w 2 or more different elements that chemically combine to form a new substance w properties differ from those of individual elements w EX: salt (Na. Cl), H 2 O

2. Mixtures ö Material that contains 2 or more substances that are not chemically combined w Can physically separate w 2 Types: Homogeneous and Heterogeneous

A. Homogeneous ö Homogeneous Mixture (Solution) w even distribution of components w very small particles w particles never settle w Can’t filter out particles w EX: saline solution, Cofffee w/ sugar

Solutions ö In a solution you have a solute and a solvent ö Solvent - the liquid the substance dissolves in w Ex: water ö Solute - what dissolves w Ex: salt

B. Heterogeneous ö Heterogeneous Mixture w Materials are not evenly blended w 2 types: colloids and suspensions w EX: granite, pizza, dry soup mix

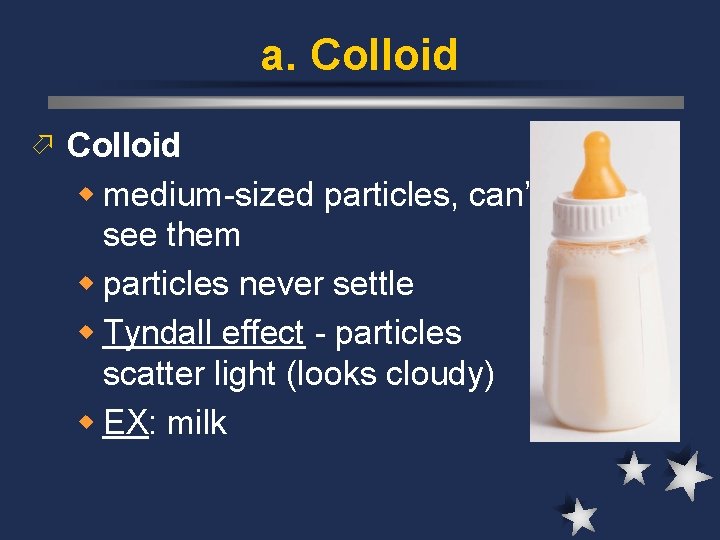
a. Colloid ö Colloid w medium-sized particles, can’t see them w particles never settle w Tyndall effect - particles scatter light (looks cloudy) w EX: milk

B. Suspension ö Suspension w large particles w particles scatter light w particles will settle out (needs to be shaken) w EX: vinaigrette dressing, muddy water, anything you have to shake

Tyndall Effect ö When light bends around particles in a mixture ö Used to tell is a mixture is homogeneous (solution) or heterogeneous (colloid or suspension) suspension ö EX: when sunlight comes through window


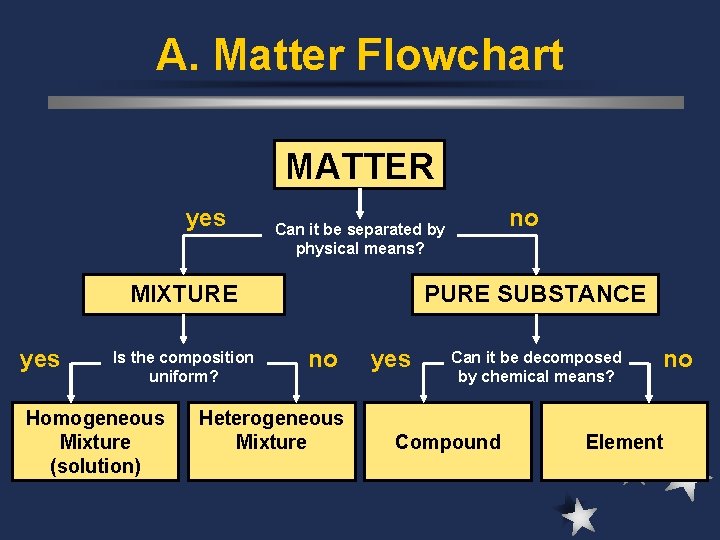
A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no Can it be separated by physical means? PURE SUBSTANCE no Heterogeneous Mixture yes Can it be decomposed by chemical means? Compound no Element

Law of Conservation of Mass ö Matter is neither created or destroyed in a chemical change ö The mass of all substances before the reaction = mass after the reaction ö 2 H 2 O 2 2 H 2 O + O 2