Classification of Matter Classification of Matter Pure Substance

Classification of Matter
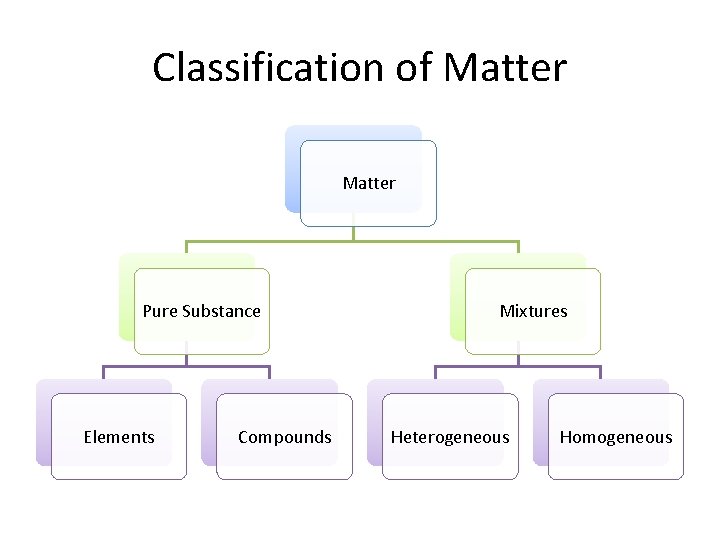
Classification of Matter Pure Substance Elements Compounds Mixtures Heterogeneous Homogeneous
(Pure) Substances • In a substance, all particles (atoms or molecules) are the same • Has a definite composition (always the same ‘recipe’) • Has a unique set of molecules
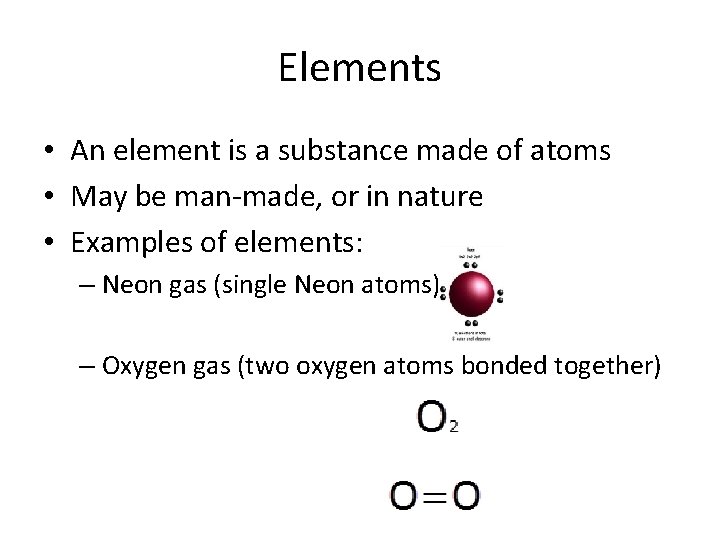
Elements • An element is a substance made of atoms • May be man-made, or in nature • Examples of elements: – Neon gas (single Neon atoms) – Oxygen gas (two oxygen atoms bonded together)
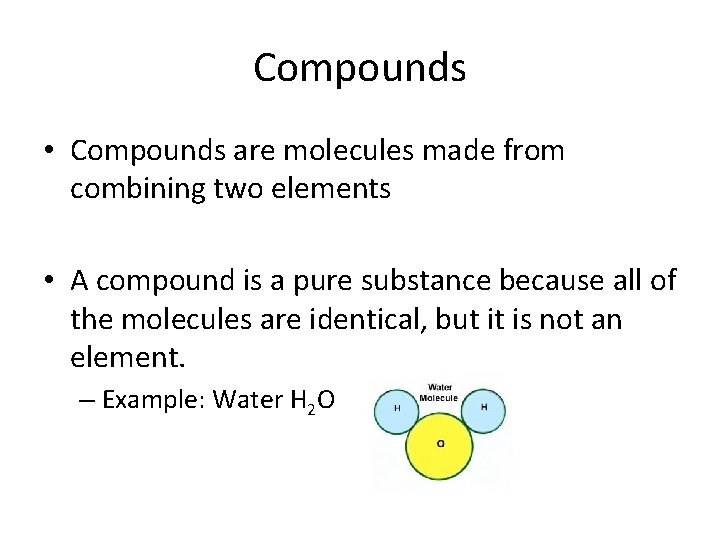
Compounds • Compounds are molecules made from combining two elements • A compound is a pure substance because all of the molecules are identical, but it is not an element. – Example: Water H 2 O

Mixtures In a mixture, • Two or more substances • Composition is not uniform • Not chemically bonded
Heterogeneous Mixture • Visibly different particles – Examples: • Dressing has visible bits of seasoning, droplets of oil and water • Ocean has water, salt, algae, fine sand particles, living cells

Homogeneous Mixture • All particles of all types present are the same; you cannot separate – Example: Sugar dissolved in water

Homogeneous Mixtures are also called ‘Solutions’ • Can be a liquid or a gas or a solid • A solution may be a compound (such as water) • Or a homogeneous mixture – Examples: • Air, which is made up of oxygen, nitrogen, carbon dioxide, water vapor, and other gases • Stainless steel, which is made up of a mixture of metals (iron, chromium, nickel)
- Slides: 9