Classification of Matter Chapter 1 Chemistry 2 A











- Slides: 25

Classification of Matter Chapter 1 Chemistry 2 A

• Chemistry: the field of study concerned with the characteristics, composition, and transformations of matter • Matter: anything that has mass and occupies space – Living and non-living – Macroscopic and microscopic

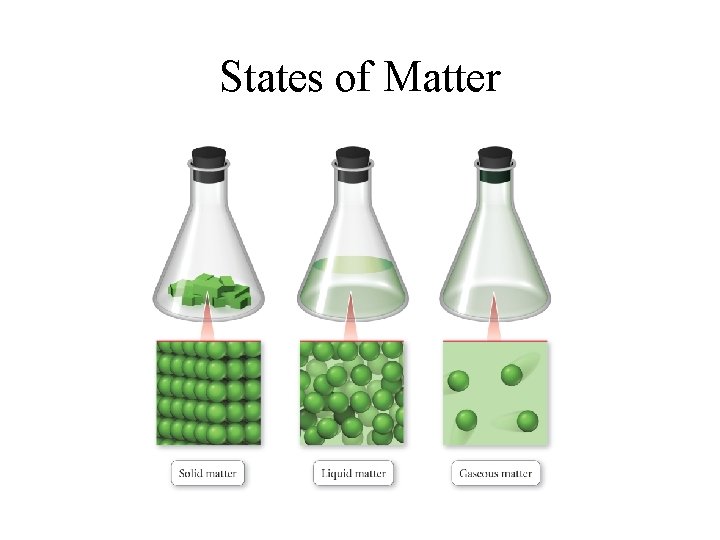
States of Matter

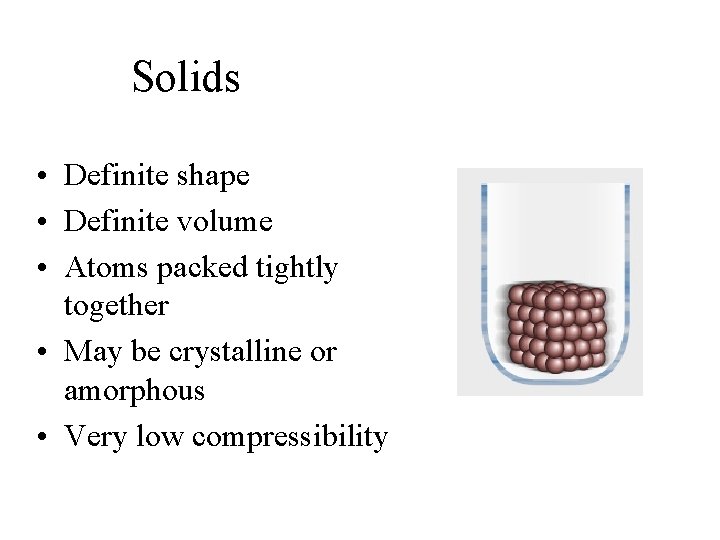
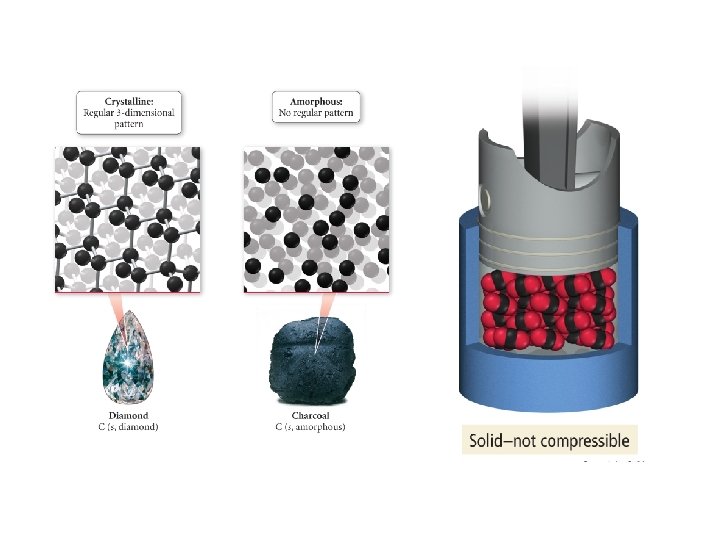
Solids • Definite shape • Definite volume • Atoms packed tightly together • May be crystalline or amorphous • Very low compressibility


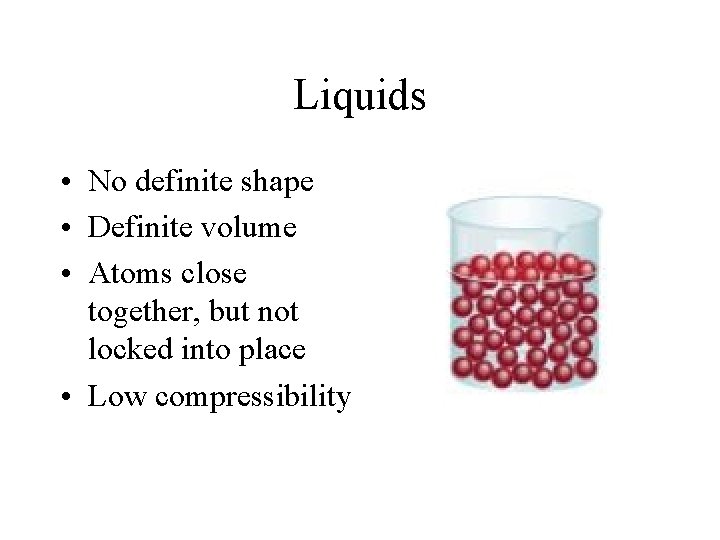
Liquids • No definite shape • Definite volume • Atoms close together, but not locked into place • Low compressibility

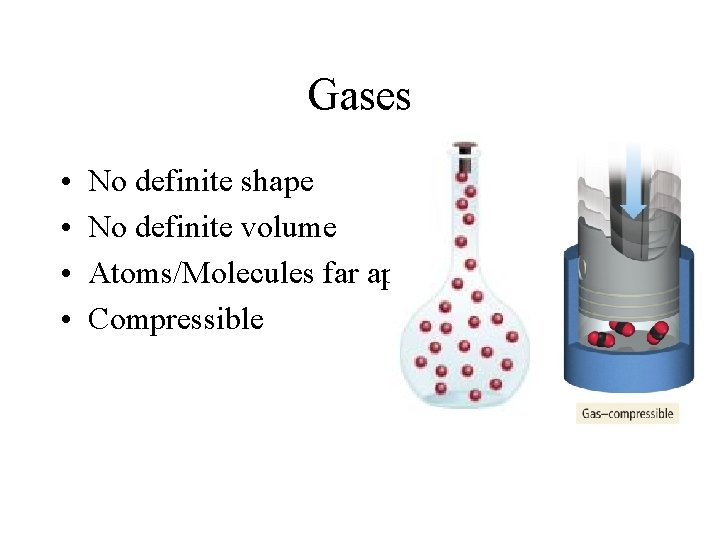
Gases • • No definite shape No definite volume Atoms/Molecules far apart Compressible

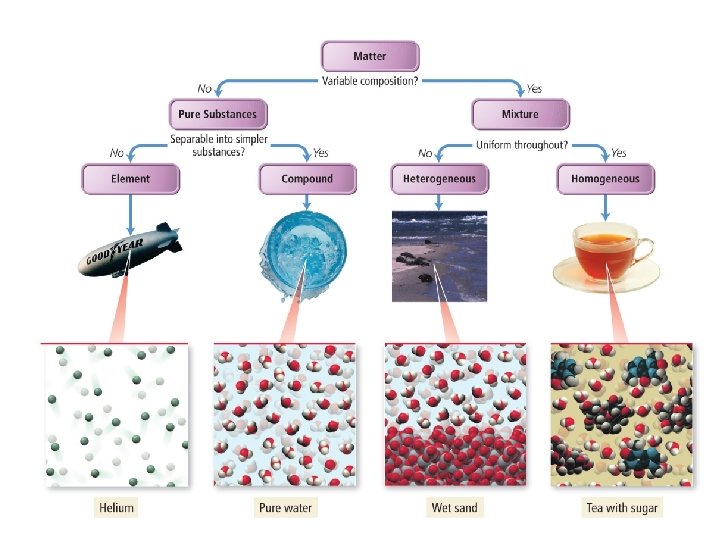
Pure Substances 1) Element: a substance that cannot be decomposed or transformed into other chemical substances by ordinary chemical processes • Atom: smallest particle of an element that can exist and still have the properties of that element – Aluminum (Al), Carbon (C), Neon (Ne), Potassium (K)



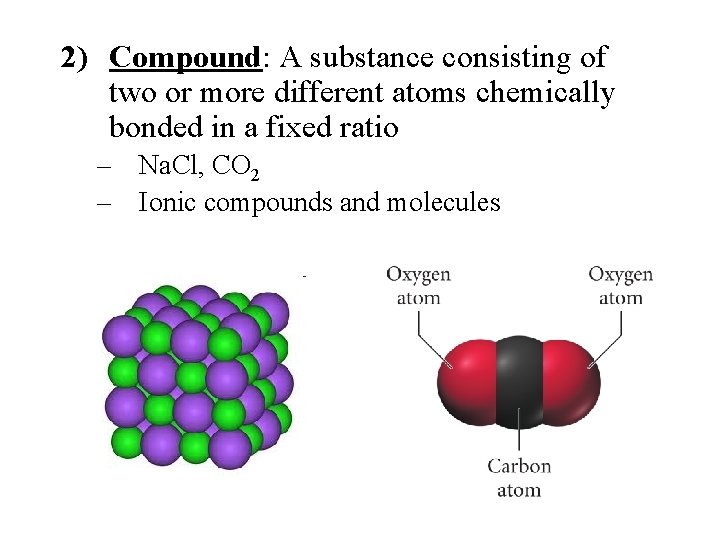
2) Compound: A substance consisting of two or more different atoms chemically bonded in a fixed ratio – Na. Cl, CO 2 – Ionic compounds and molecules

Mixtures • Mixtures: combinations of two or more pure substances in which each substance retains its own identity 1) Heterogeneous Mixture: a substance in which elements and/or compounds are blended together in such a way that there is no uniform composition or fixed ratio of the components of the mixture • Examples: Oil and water, mixed nuts

2) Homogeneous Mixture: A substance in which the different elements/compounds being mixed exist in definite ratios, but are not chemically bonded – – Consists of two or more substances in the same phase No amount of magnification will reveal an interface Called a “solution” Examples: Salt water, sugar water, O 2 dissolved in water


Problems • 1) 2) 3) 4) 5) 6) Decide whether the following mixtures are heterogeneous or homogenous Chocolate chip cookie dough Wine Milk O 2 in water Chicken noodle soup Kool-Aid

Physical Properties • Properties of an object or substance that can be measured or perceived without changing the identity of the substance

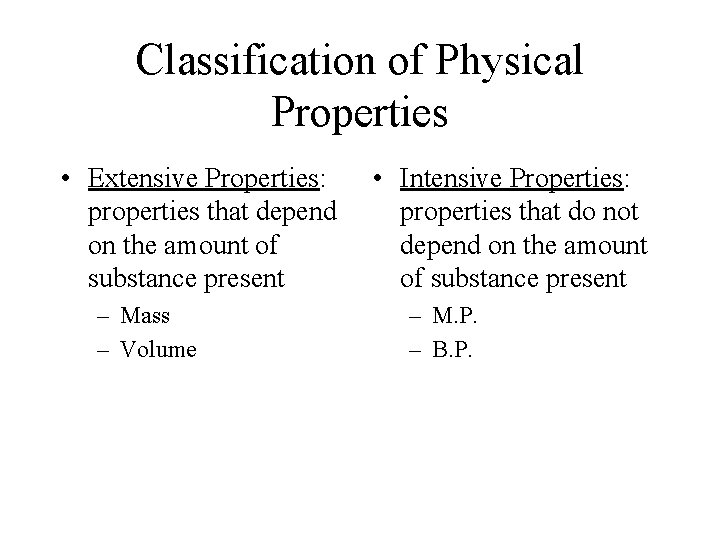
Classification of Physical Properties • Extensive Properties: properties that depend on the amount of substance present – Mass – Volume • Intensive Properties: properties that do not depend on the amount of substance present – M. P. – B. P.

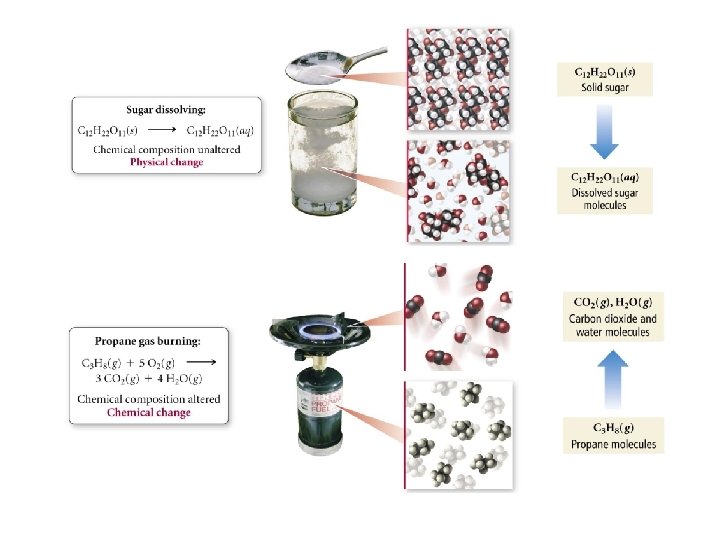
Physical Change • A change in a physical property of a substance • Same substance before and after the change

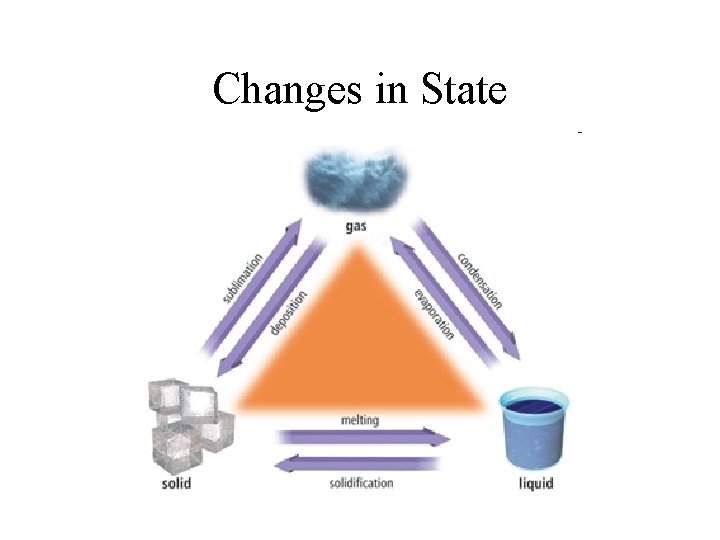
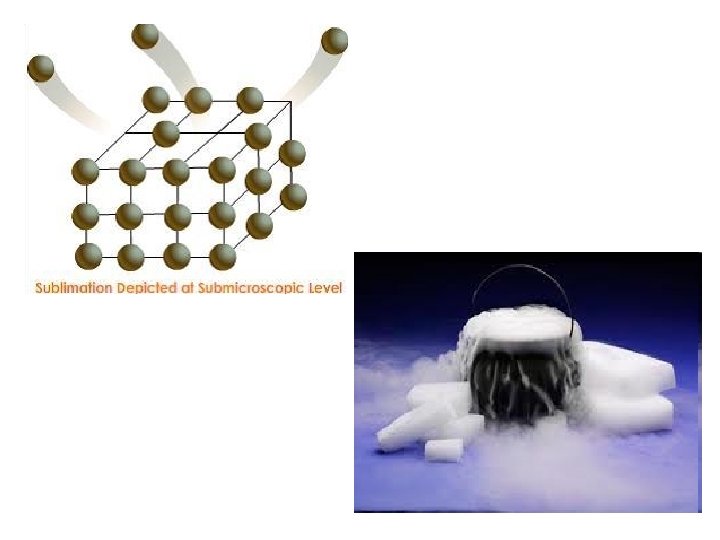
Changes in State


Chemical Property • Any property of a material that becomes evident during a chemical reaction – Qualities that become evident by changing a substance’s identity • Capability to undergo chemical reactions – – Flammability Acidity Corrosiveness Toxicity

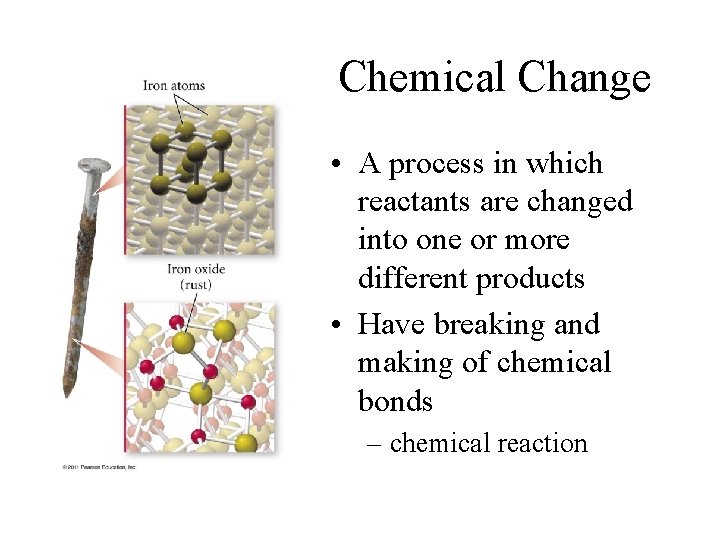
Chemical Change • A process in which reactants are changed into one or more different products • Have breaking and making of chemical bonds – chemical reaction



Problems • 1) 2) 3) 4) 5) Decide whether the following are chemical or physical changes Sawing a log in half Melting chocolate in a pot on your stove Burning your chocolate Dissolving a nickel in acid Cutting your hair