Classification of Matter Chapter 1 Can it be

Classification of Matter Chapter 1

Can it be separated by physical means? YES MIXTURE A combination of 2 or more substances, each retaining its properties NO SUBSTANCE – Homogeneous matter with same composition and properties throughout

For a mixture, is its composition uniform? YES NO HOMOGENEOUS HETEROGENEOUS Evenly mixed; same composition throughout; a solution Unevenly mixed; variable composition

For a heterogeneous mixture, will its particles separate or settle out? YES SUSPENSION NO COLLOID
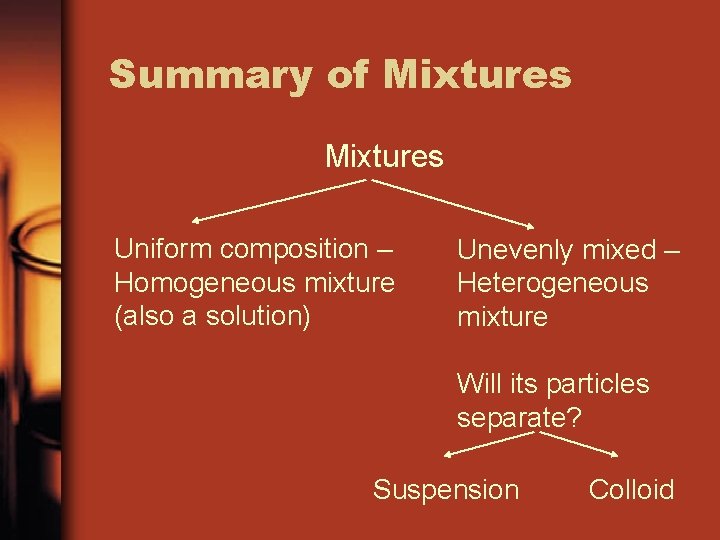
Summary of Mixtures Uniform composition – Homogeneous mixture (also a solution) Unevenly mixed – Heterogeneous mixture Will its particles separate? Suspension Colloid

Examples of Mixtures classify each as homo- or hetero- • • Kool-Aid Granite Air in this room Tossed salad Syrup Stainless steel Salt water Chocolate chip cookie
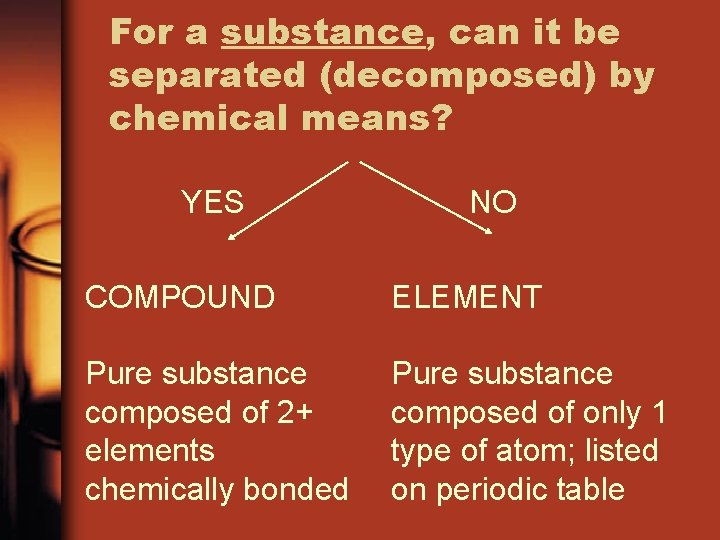
For a substance, can it be separated (decomposed) by chemical means? YES NO COMPOUND ELEMENT Pure substance composed of 2+ elements chemically bonded Pure substance composed of only 1 type of atom; listed on periodic table
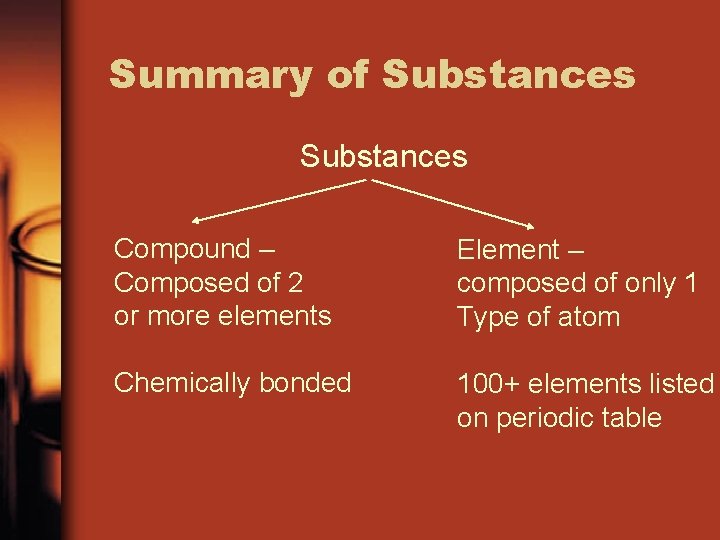
Summary of Substances Compound – Composed of 2 or more elements Element – composed of only 1 Type of atom Chemically bonded 100+ elements listed on periodic table

Examples of Substances classify as compound or element • Oxygen • Water • Carbon dioxide • Iron • Sodium chloride (table salt) • Hydrogen • Uranium • Glucose
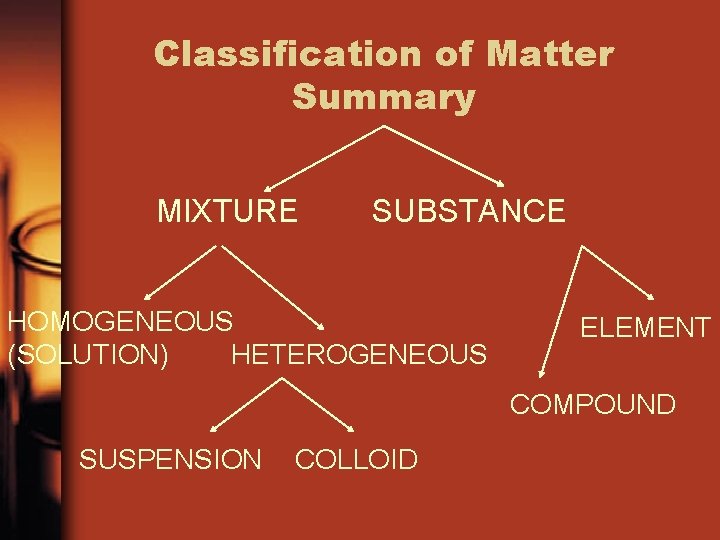
Classification of Matter Summary MIXTURE SUBSTANCE HOMOGENEOUS (SOLUTION) HETEROGENEOUS ELEMENT COMPOUND SUSPENSION COLLOID
- Slides: 10