Classification of Matter C 2 3 Matter can











- Slides: 14

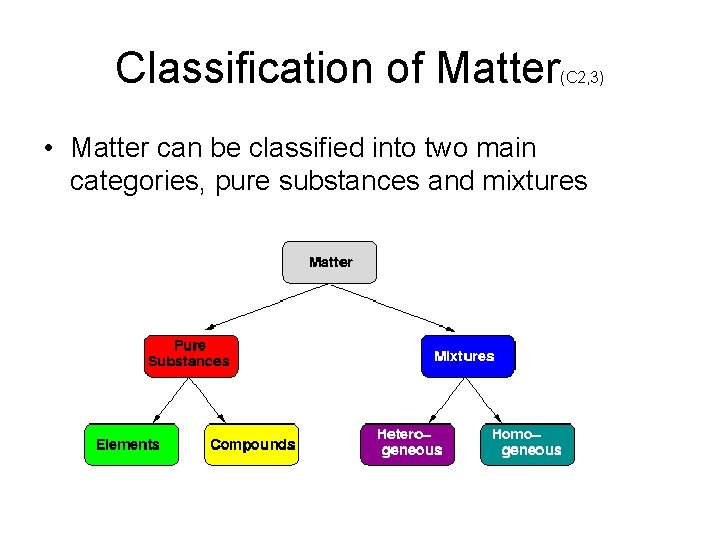
Classification of Matter (C 2, 3) • Matter can be classified into two main categories, pure substances and mixtures

Pure Substances • Pure substances one type of particle. – Element one type of atom • examples: – gold, silver, lead, and mercury. – Molecules & Compounds two or more types of atoms joined chemically. • examples: – water (H 2 O), carbon dioxide (CO 2) & sodium chloride (Na. Cl) – Molecules & Compounds can only be separated by chemical means.

Mixtures • Mixtures: – loose arrangements of materials. – contains two or more substances that are not chemically joined – easily separated into its various components by physical means, such as filtration, distillation (boiling) or extraction. – each component retains its own identity, – ratio of the components can change. – Mixtures can be further classified into • two categories homogeneous & heterogeneous

• Homogeneous mixtures – One phase: • Uniform - the composition of the mixture is the same throughout the mixture on a microscopic level. • Example salt water. • Homogeneous mixtures may look like pure substances due to their uniformity, but they are not. . • Heterogeneous mixture: – More than one phase: • Composition NOT uniform • Various components visible. • Example potting soil, oil & vinegar salad dressing.

Solutions • Solutions are : – Transparent with different compositions. – Made of: • a solute (the substance that dissolves) and • a solvent (the substance that does the dissolving). – When the solvent is able to dissolve a solute, the solute is soluble. – When the solvent is unable to dissolve the solute, the solute is insoluble.

Alloys • Alloys are: – Homogeneous mixtures of two or more metals. – Very useful often having better properties than those of the pure metals that they are made of. • Iron is relatively soft & rusts, – Add carbon & you get steel stronger. – Add nickel & chromium also is stainless steel (strong no rust) • Gold is soft – Add copper to make jewelry – Gold is beautiful & lustrous, copper adds strength.

Mechanical Mixtures • Mechanical mixtures are: – Heterogeneous, (can see the different particles). – Divided into three categories (based on the size of the particles). • Ordinary mechanical mixtures: – Different parts large & visible – Ex: A sand salt.

Suspensions • Suspension: – Particles may be seen (eye or microscope). – If undisturbed, gravity will eventually cause the suspended particles to separate. – An example of a suspension is dirty river water - after awhile, the dirt settles. – Emulsifiers prevent suspensions from separating • Ex – egg in oil or vinegar – Flour in gravy Are suspensions heterogeneous or homogeneous?

Colloids • Colloid – particles too small to see (even with a microscope) – gravity will not cause them to separate. – Colloids are heterogeneous but appear homogenous. – A colloid will cause a beam of light to be scattered or bent. • Tyndall Effect


Properties of Matter Physical & Chemical Physical Properties of Matter • Physical properties describe what the material is like. These are visible features that can be observed (qualitative) or measured (quantitative). – – – – State – solid, liquid, gas, plasma Colour Density, viscosity Hardness, brittleness Taste, odour, texture, lustre, clarity Melting and boiling points Ductility (bendable) and malleability (able to be hammered)

Chemical Properties of Matter • Chemical properties explain how a material behaves and reacts in relation to other materials. – Types of bonds – Reactivity – Ions formed

Changes in Matter Physical & Chemical Physical change. • A physical change when there is no new product formed. The particles of the starting substance are not changed. A physical change can easily be reversed.

Chemical Change • In a chemical change, a new substance is formed. Clues that a Chemical Change Took Place §A new colour appeared §Heat or light was given off §Bubbles of gas were given off §A precipitate formed §The change is difficult to reverse