Classification of Matter and KMT Whats the Matter









- Slides: 17

Classification of Matter and KMT

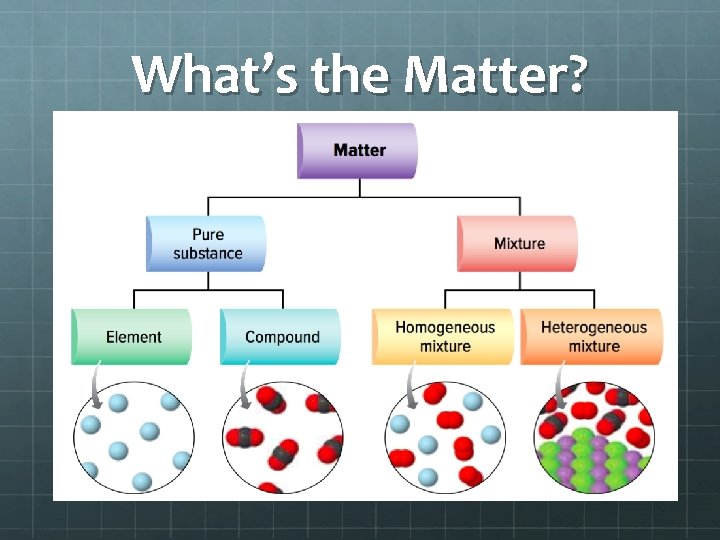
What’s the Matter? Matter – is anything that has mass or volume. Mass - is the amount of matter that an object has. Volume – is the amount of space that an object fills. Ultimately, matter is the material that makes up the whole universe.

What’s the Matter What kind of matter do you see in this image? What kind of matter exits in this image? Does a classroom have matter? What kind?

What’s the Matter?

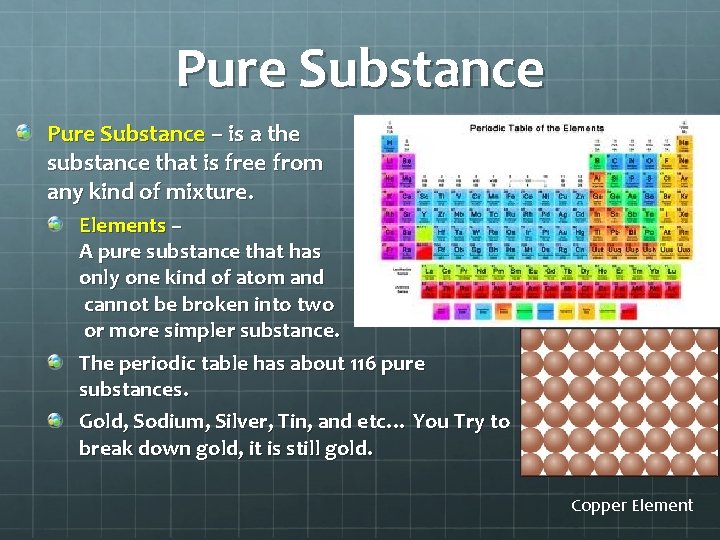
Pure Substance – is a the substance that is free from any kind of mixture. Elements – A pure substance that has only one kind of atom and cannot be broken into two or more simpler substance. The periodic table has about 116 pure substances. Gold, Sodium, Silver, Tin, and etc… You Try to break down gold, it is still gold. Copper Element

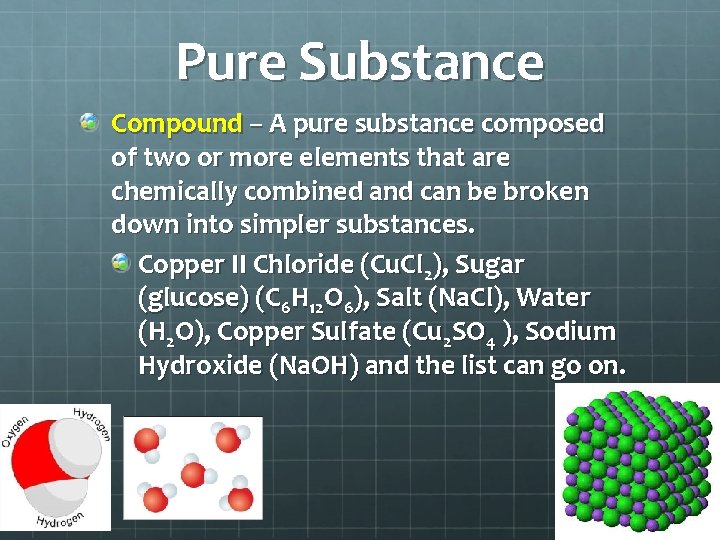
Pure Substance Compound – A pure substance composed of two or more elements that are chemically combined and can be broken down into simpler substances. Copper II Chloride (Cu. Cl 2), Sugar (glucose) (C 6 H 12 O 6), Salt (Na. Cl), Water (H 2 O), Copper Sulfate (Cu 2 SO 4 ), Sodium Hydroxide (Na. OH) and the list can go on.

Mixtures – when two or more substances are combined where the particles are not chemically joined. Homogeneous Mixture – is when the components that make up the mixture are uniformly distributed throughout the mixture. There is only one phase of matter. I. E. you would not see both a liquid and a solid or a liquid and a gas. Pop is a mixture of sugar, corn syrup, and carbon dioxide gas in water. All of it is evenly mixed so as to have the appearance of one substance, yet not chemically joined. . air (nitrogen, oxygen, hydrogen), steel (iron and other metallic elements), sugar water (water mixed with a sugar)

Mixtures Heterogeneous Mixture – mixture where the component of the mixture are not uniform. There can be many different phases of matter. I. E. you could have both a liquid and a solid, or a liquid and a gas. Cereal and milk, pizza, salad dressing, soup with chives, taco.

Kinetic Molecular Theory – is the idea that matter is made from moving invisible particles. The following are the principals of Kinetic Molecular Theory: All matter is made up of tiny particles Different substances have different particles The particles are in constant motion The more energy the particles have, the faster they move. The attraction between particles decreases with an increase in distance.

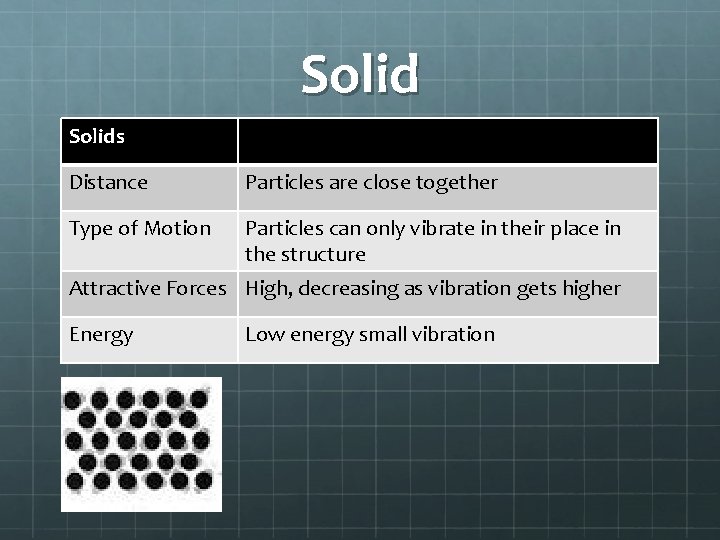
Solids Distance Particles are close together Type of Motion Particles can only vibrate in their place in the structure Attractive Forces High, decreasing as vibration gets higher Energy Low energy small vibration

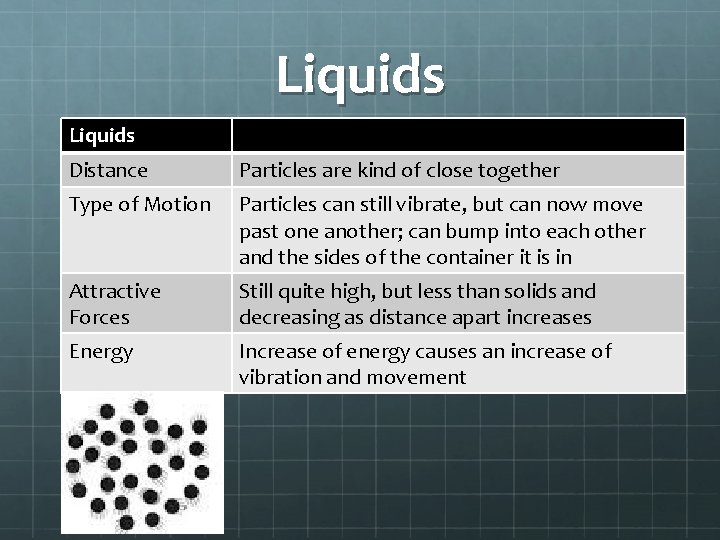
Liquids Distance Particles are kind of close together Type of Motion Particles can still vibrate, but can now move past one another; can bump into each other and the sides of the container it is in Attractive Forces Still quite high, but less than solids and decreasing as distance apart increases Energy Increase of energy causes an increase of vibration and movement

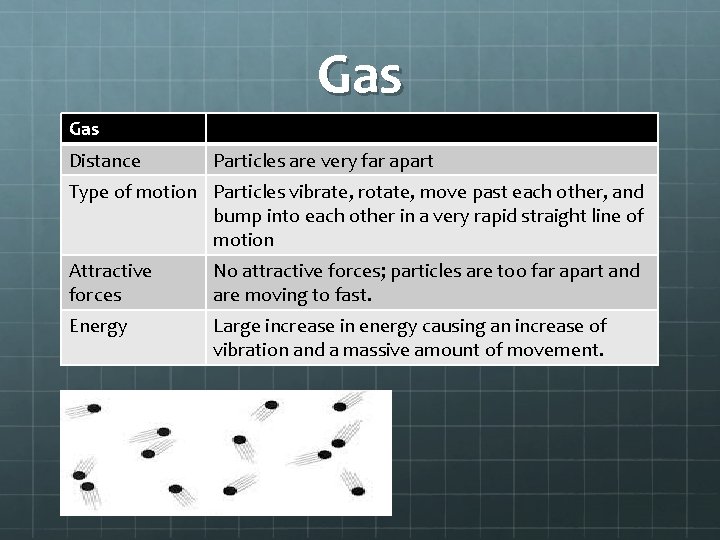
Gas Distance Particles are very far apart Type of motion Particles vibrate, rotate, move past each other, and bump into each other in a very rapid straight line of motion Attractive forces No attractive forces; particles are too far apart and are moving to fast. Energy Large increase in energy causing an increase of vibration and a massive amount of movement.

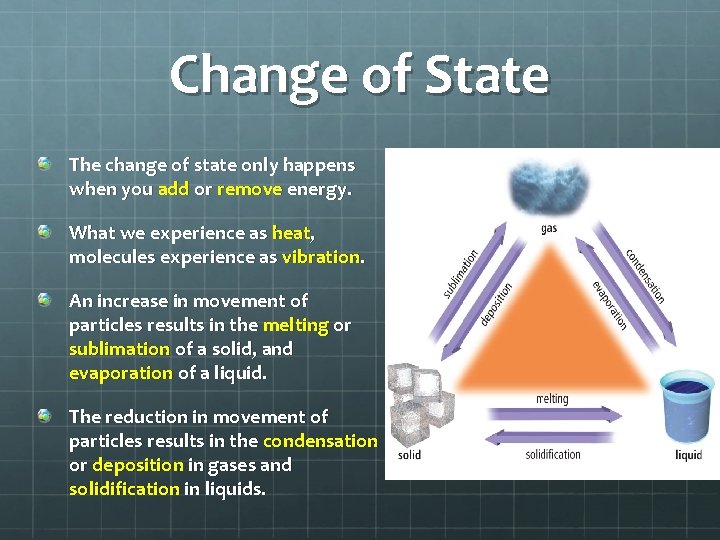
Change of State The change of state only happens when you add or remove energy. What we experience as heat, molecules experience as vibration. An increase in movement of particles results in the melting or sublimation of a solid, and evaporation of a liquid. The reduction in movement of particles results in the condensation or deposition in gases and solidification in liquids.

Explain Dissolving The kinetic molecular theory perfectly describes how compounds, like sugar, dissolve in water. When water is in a liquid state, the molecules are vibrating and have space between them for sugar to enter into the water and dissolve. If you increase the heat of the water, the vibration of the water molecule increases, thus more space is created between the water molecules. Therefore, sugar has more space to enter in between the water molecules and the sugar will dissolve a lot faster.

Explain Density Problem, why is it that the density of water in solid form has a different density when it is in liquid form and a different density when it is in a gas form? Answer, the kinetic molecular theory. When you add energy to a particle and increase the vibration of the particle, the particles in the compound expand thus the volume changes. If the volume increases and the mass stays the same, the density decreases.

Finally…. Ever notice on sidewalks there are gaps between the concrete sidewalk? Well kinetic molecular theory explains why we need this. If a sidewalk was one solid strip without any gaps, during cooler months the sidewalk contracts and during warmer months the sidewalk expands. This constant motion of contraction and expansion leads to the sidewalk cracking. If you put in gap when laying the concrete, you mitigate and minimize the amount of cracks that will be created.

Assignment Page 156 Textbook – 10 Page 175 Textbook – 4 - 8