Classification of Matter Activity and Notes Objective Today

Classification of Matter Activity and Notes

Objective Today I will be able to: Apply knowledge of the intro to chemistry unit to an exam review Differentiate between elements, compounds and mixtures by completing a close reading Differentiate between a element, compound, homogeneous or heterogeneous mixture Informal assessment – monitor student interactions as they complete the practice Formal assessment – analyze student responses to the exit ticket and concept maps Common core connection Build Strong Content Knowledge Value Evidence Reason abstractly and quantitatively

Lesson Sequence Evaluate: Warm-up Evaluate: Exam Review Activity Explain: Element, compound mixture close reading Explain: Classification of Matter Notes Elaborate: Element, Compound Mixture Practice Evaluate: Exit Ticket

Dimensional Analysis the Crazy Warm Up 1 terrier = ______ kangaroos These are your conversion factors: 1 terrier = 4 hippos 17 hippos = 2 kangaroos

925 bears = _______ terriers 1 terrier = 4 hippos 4 hamsters = 6 bears 17 hippos = 2 kangaroos 2 hamsters = 3 kangaroos

4031 pickles = _____ funnel cake 32 funnel cakes = 4 corn dogs 980 pickles = 1 cotton candy 96 cotton candy = 55 corn dogs

Objective Today I will be able to: Apply knowledge of the intro to chemistry unit to an exam review Differentiate between elements, compounds and mixtures by completing a close reading Differentiate between a element, compound, homogeneous or heterogeneous mixture

Homework Study for Quest!

Agenda Warm-up Exam Review Activity Element, compound mixture close reading Classification of Matter Notes Element, Compound, Mixture Practice Exit Ticket

Review Activity Write one question for each topic on the exam Lab Safety Accuracy and Precision Scientific Notation (Calc) Sig Figs (Calc) Dimensional Analysis (Calc) Make an answer key on a separate sheet of paper Trade and take a partner’s quiz

Element, Compound, Mixtures Close Reading Use the textbooks in the classroom to complete the worksheet

Draw this chart on a piece of notebook paper Matter Element Compound Mixture Definition Real life example picture

Classification of Matter Notes
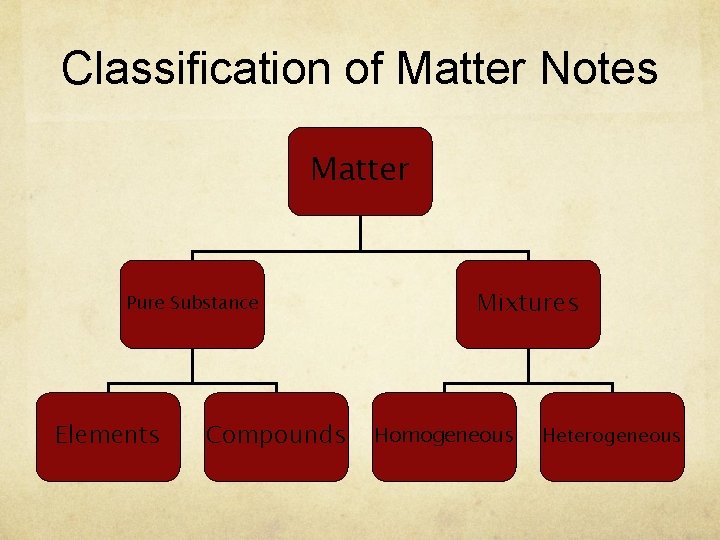
Classification of Matter Notes Matter Pure Substance Elements Compounds Mixtures Homogeneous Heterogeneous

Mixtures

Mixtures n Two or more substances together which are NOT chemically combined n Properties - Substances keep separate identities and properties - Substances may be present in any amount - Substances can be separated by simple physical means (filtering, magnet, etc)

Mixtures n Heterogeneous - not the same throughout (trail mix, bird seed) - Particles are large enough to be seen - Mixtures separate on standing

Mixtures Homogeneous - the same throughout (salt water) - Particles are small and not easily recognized – uniform mixture of particles - Does not settle on standing
Mixtures Solution – example of a homogeneous mixture - one substance dissolved in another (lemonade, ocean water, gold jewelry) - Particles are very small and evenly spread out - Cannot be separated by simple physical

Ways to Separate Mixtures Filtration Evaporation Chromatography Distillation

Pure Substances

Elements n Pure substance that cannot be broken down into simpler substances by ordinary chemical means (Iron, Copper, Tungsten)

Elements n All matter is composed of elements n Have definite properties n Made up of one type of atom n Periodic Table organizes elements according to their properties

Compounds Two or more different elements combine in a chemical reaction New substances with new properties are formed Elements combine in fixed proportions - water (H 2 O) always has two hydrogen atoms and one oxygen atom

Compounds Examples of Fixed Proportions C 3 H 7 OH - Propanol - 3 carbon, 8 hydrogen, 1 oxygen (NH 4)2 O – Ammonium Oxide - 2 nitrogen, 8 hydrogen, 1 oxygen

Compounds Two elements = binary compound (CO 2) Three elements = ternary compound (C 6 H 12 O 6)

Element Compound Mixture Practice Complete the practice at your desk. Problems not completed in class will be completed for HW
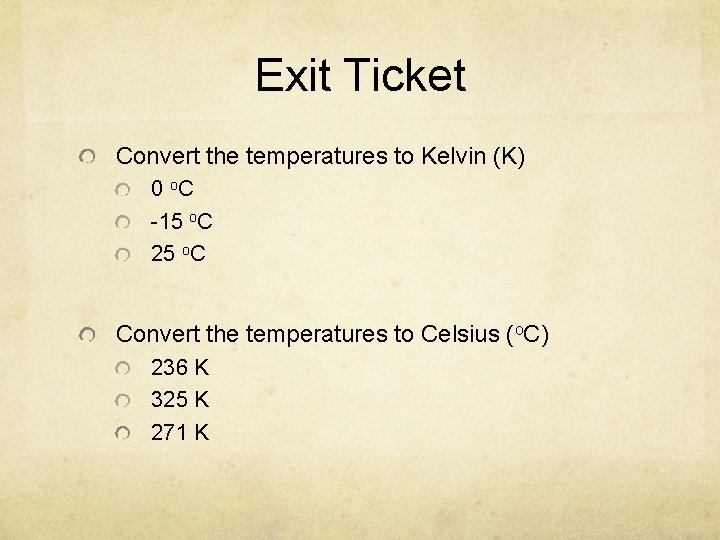
Exit Ticket Convert the temperatures to Kelvin (K) 0 o. C -15 o. C 25 o. C Convert the temperatures to Celsius (o. C) 236 K 325 K 271 K
- Slides: 28