Classification of Chemical Reactions Types of Chemical Reactions








- Slides: 15

Classification of Chemical Reactions

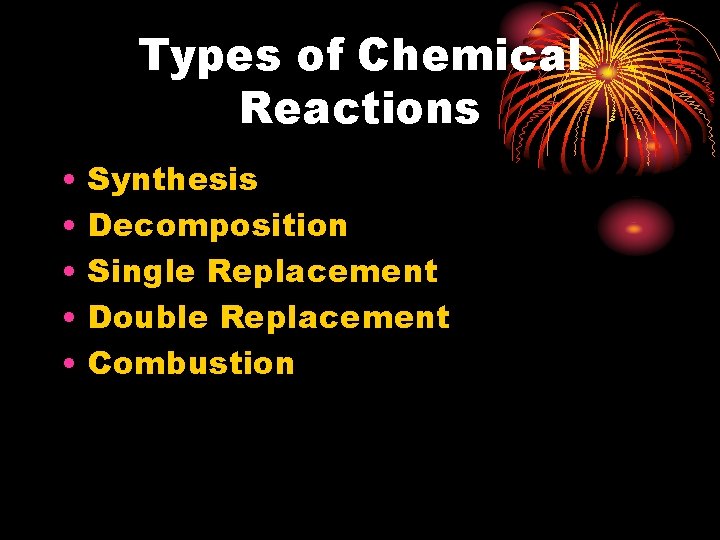
Types of Chemical Reactions • • • Synthesis Decomposition Single Replacement Double Replacement Combustion

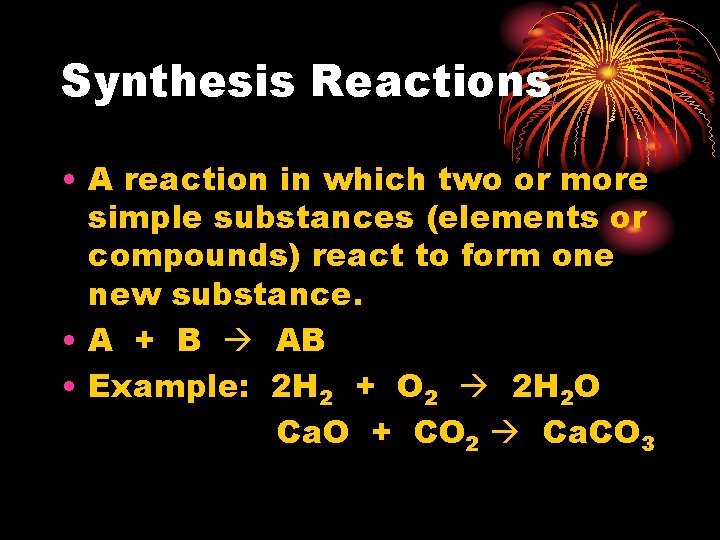
Synthesis Reactions • A reaction in which two or more simple substances (elements or compounds) react to form one new substance. • A + B AB • Example: 2 H 2 + O 2 2 H 2 O Ca. O + CO 2 Ca. CO 3

Decomposition Reactions • A reaction in which one substance breaks down into two or more simple substances • AB A + B • Example: 2 H 2 O 2 2 H 2 O + O 2 2 KCl. O 3 2 KCl + 3 O 2

Single Replacement Reactions • A reaction in which an uncombined element replaces another element in a compound • A + BC AC + B • “Like replaces like” – metals replace metals and nonmetals replace nonmetals

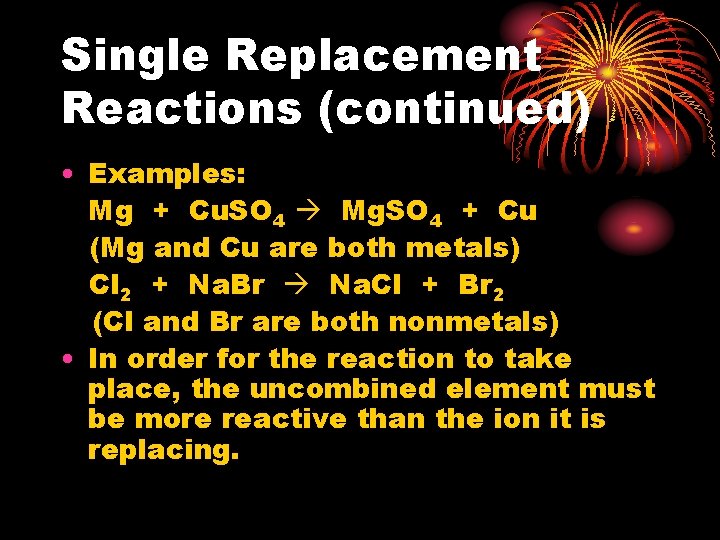
Single Replacement Reactions (continued) • Examples: Mg + Cu. SO 4 Mg. SO 4 + Cu (Mg and Cu are both metals) Cl 2 + Na. Br Na. Cl + Br 2 (Cl and Br are both nonmetals) • In order for the reaction to take place, the uncombined element must be more reactive than the ion it is replacing.

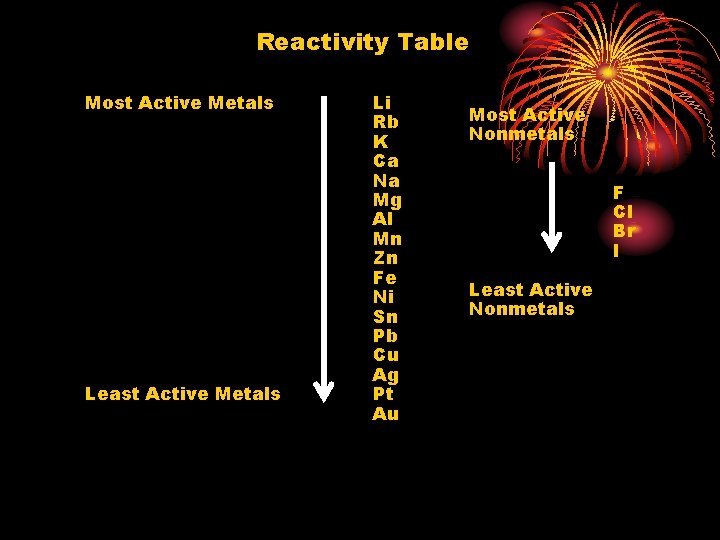
Reactivity Table Most Active Metals Least Active Metals Li Rb K Ca Na Mg Al Mn Zn Fe Ni Sn Pb Cu Ag Pt Au Most Active Nonmetals F Cl Br I Least Active Nonmetals

Which of the following reactions can take place? I 2 + Na. F F 2 + Na. I Mg + HCl Mg. Cl 2 + H 2 Cu + Mg. SO 4 Cu. SO 4 + Mg K + Na. OH KOH + Na

Double Replacement Reactions • A reaction in which two positive ions replace each other in compounds • AB + CD AD + CB • In order for this type of reaction to occur, one of the following must happen: a precipitate is formed, a gas is formed, or one of the products must be covalent.

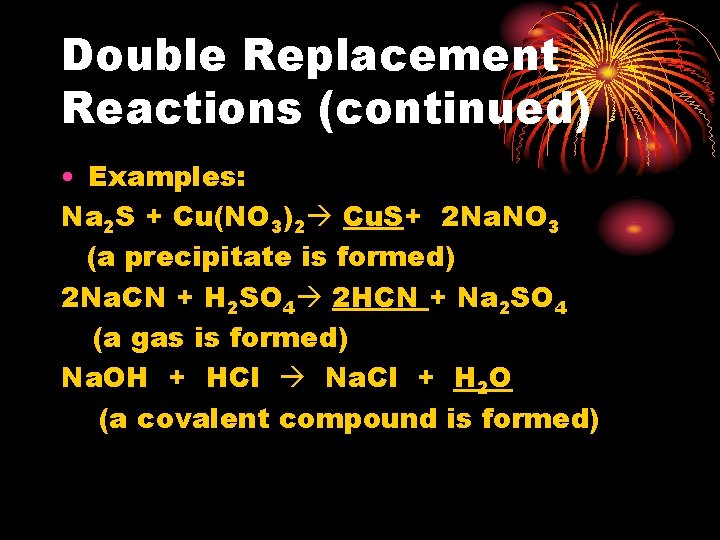
Double Replacement Reactions (continued) • Examples: Na 2 S + Cu(NO 3)2 Cu. S+ 2 Na. NO 3 (a precipitate is formed) 2 Na. CN + H 2 SO 4 2 HCN + Na 2 SO 4 (a gas is formed) Na. OH + HCl Na. Cl + H 2 O (a covalent compound is formed)

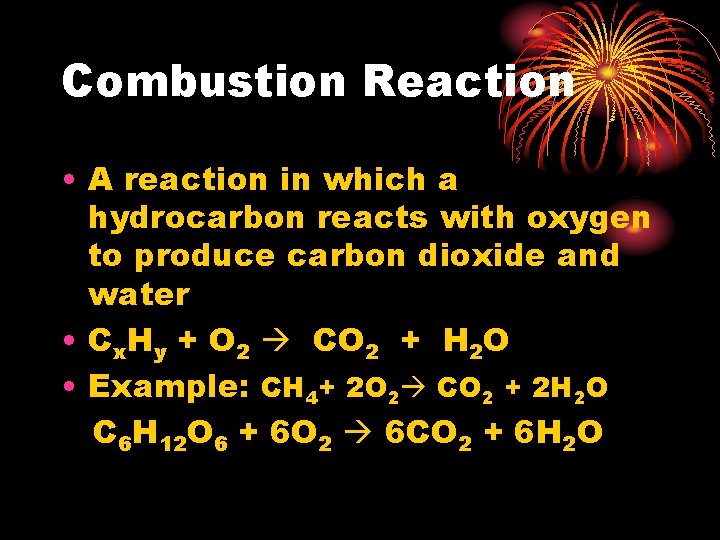
Combustion Reaction • A reaction in which a hydrocarbon reacts with oxygen to produce carbon dioxide and water • Cx. Hy + O 2 CO 2 + H 2 O • Example: CH 4+ 2 O 2 CO 2 + 2 H 2 O C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O

Review Classify each of the following reactions by type and then balance the equation: Pb(NO 3)2 + K 2 Cr. O 4 Pb. Cr. O 4+ KNO 3 Cl 2 + KI KCl + I 2 C 3 H 6 + O 2 CO 2 + H 2 O Al(OH)3 Al 2 O 3 + H 2 O Li + O 2 Li 2 O HCl + Fe 2 O 3 Fe. Cl 3 + H 2 O Mg. CO 3 Mg. O + CO 2 Ba(CN)2 + H 2 SO 4 HCN + Ba. SO 4

Classifying Reactions Based on Energy Changes • Exothermic reactions are reactions in which heat/energy is released • Example: 2 C 4 H 10 + 13 O 2 8 CO 2 + 10 H 2 O • The container often feels warm during exothermic reactions • reactions involving explosions and burning are examples

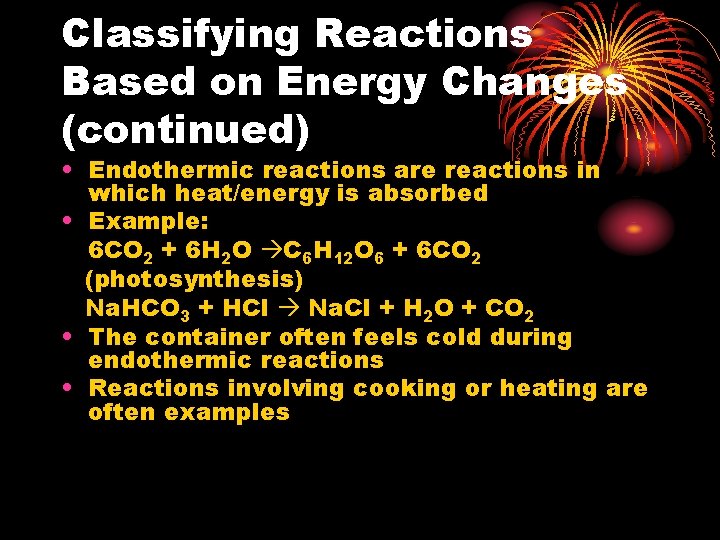
Classifying Reactions Based on Energy Changes (continued) • Endothermic reactions are reactions in which heat/energy is absorbed • Example: 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 CO 2 (photosynthesis) Na. HCO 3 + HCl Na. Cl + H 2 O + CO 2 • The container often feels cold during endothermic reactions • Reactions involving cooking or heating are often examples

Review • Identify each of the following as either exothermic or endothermic: Baking a cake Exploding dynamite Burning a piece of paper A chemical ice pack Hand warmers
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical reactions classification
Chemical reactions classification Classification of chemical reactions worksheet
Classification of chemical reactions worksheet Types of redox reactions
Types of redox reactions Identify types of reactions
Identify types of reactions 4 types of chemical reactions
4 types of chemical reactions What are the 5 types of chemical reactions
What are the 5 types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Five chemical change
Five chemical change 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions



























