Classification of advanced rectal cancer Gina Brown The

Classification of advanced rectal cancer Gina Brown The Royal Marsden Hospital Imperial College www. slideshare/ginabrown 3
Developments in MRI based management of Rectal Cancer • The unimportance of nodal status • The importance of extramural depth of spread as a biomarker • Recognition and effective preoperative treatment of mr. CRM involvement • Recognition and effective preoperative treatment of mr. EMVI – impact on survival • Staging and assessment of low rectal cancer

Developments in MRI based management of Rectal Cancer • Advanced Rectal Cancer Classification • Using the post treatment MRI TRG assessment as a biomarker for further preoperative treatment stratification
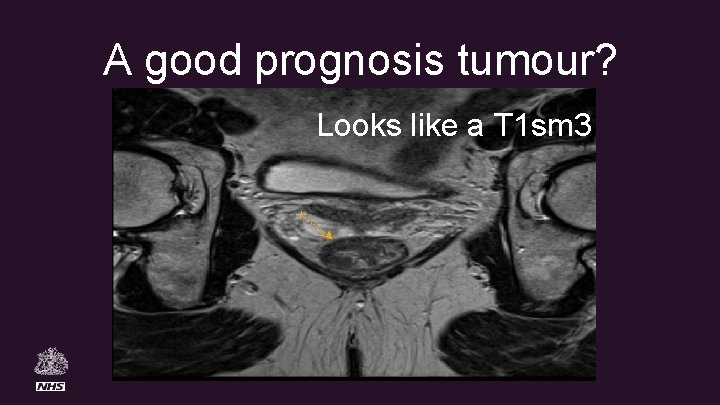
A good prognosis tumour? Looks like a T 1 sm 3
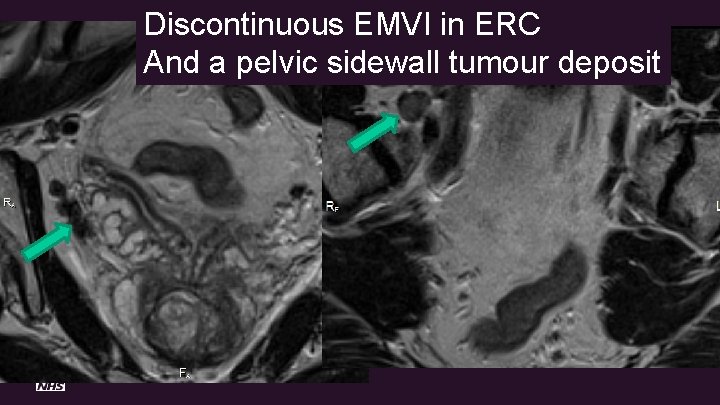
Discontinuous EMVI in ERC And a pelvic sidewall tumour deposit

Beyond TME • In a significant proportion of patients (15 -20%), tumour extends beyond the achievable TME planes and requires more extensive surgery to achieve clear margins.

Exenterative surgery • Radical resection can achieve complete tumor clearance. Reported R 0 rates range from 22%67%. • Can significantly increase survival, enhancing the prospects for long term cure. • High rate of post-operative adverse effects/morbidity. A. G. Heriot. Colorectal Dis, 2006; 8(9): 733 -747


Beyond TME Collaborative project • To demonstrate that a validated staging system can be employed, • Establish scale and scope of pelvic exenterative surgery in advanced rectal cancer beyond TME • Assess quality of life and outcomes • Develop prognostic classification to assist in counselling patients
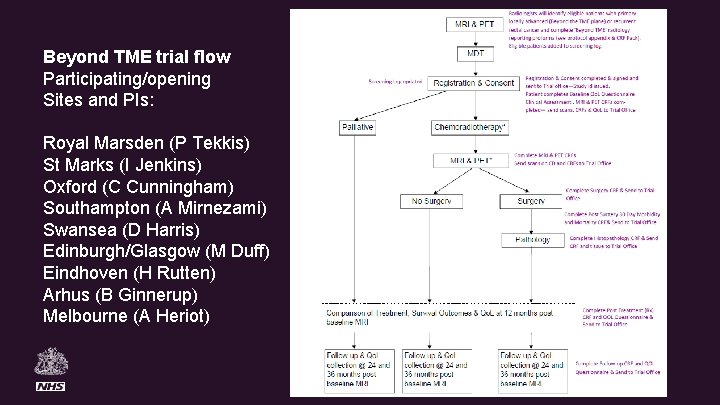
Beyond TME trial flow Participating/opening Sites and PIs: Royal Marsden (P Tekkis) St Marks (I Jenkins) Oxford (C Cunningham) Southampton (A Mirnezami) Swansea (D Harris) Edinburgh/Glasgow (M Duff) Eindhoven (H Rutten) Arhus (B Ginnerup) Melbourne (A Heriot)
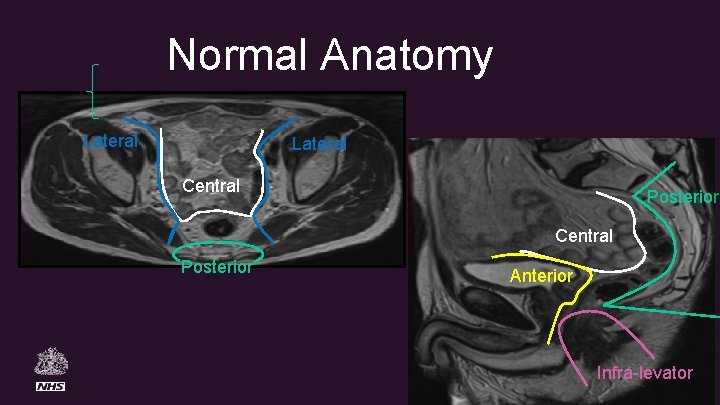
Normal Anatomy Lateral Central Posterior Anterior Infra-levator

Reporting Proforma Advanced Tumour Requiring Surgery Beyond TME Primary tumour The primary tumour is demonstrated as an [ Annular | Semi-annular | Ulcerating | | Polypoidal | Mucinous] mass with a [nodular / smooth] infiltrating border. The distal edge of the luminal tumour arises at a height of [ ] mm from anal verge: The distal edge of the tumour lies [ ]mm [Above, at, below] the top of the puborectalis sling The tumour extends craniocaudally over a distance of [ ] mm The proximal edge of tumour lies [above at below] the peritoneal reflection Invading edge of tumour extends from [ to ] O’clock Tumour is [confined to] [extends through] the muscularis propria: Extramural spread is [ ] mm mr. T stage: [T 1 ] [ T 2 ] [ T 3 a] [ T 3 b ] [ T 3 c] [ T 3 d ] [T 4 visceral ] [T 4 peritoneal] Lymph node assessment Only benign reactive and no suspicious nodes shown [N 0] [ ] mixed signal/irregular border nodes [N 1/N 2] Extramural venous invasion: [ No evidence ] [ Evidence] [ ] Small [ ]Medium [ ]Large vein invasion is present Peritoneal deposits: [ No evidence] [ Evidence] Pelvic side wall lymph nodes: [ None] [ Benign] [ Malignant mixed signal/irreg border] Location: [Obturator fossa • R • L ]. [External Iliac Nodes • R • L]. [internal iliac • R • L ]

Disease affects central compartment Above the peritoneal reflection within the pelvis Disease is present/ absent Ureters are free of disease Below the Peritoneum anteriorly Bladder /Uterus/Vagina/Ovaries Prostate/Seminal vesicles/Urethra are free of disease Posteriorly The bony cortex/periosteum from S 1 -S 2 is / is not involved by disease The bony cortex/periosteum from S 3 -S 5 /coccyx is/ is not involved by disease Presacral fascia (S 1/S 2/S 3/S 4/S 5) is not involved by disease Sciatic nerve/ S 1/S 2 nerve roots No disease Disease is present Laterally Pelvic fascia are free of disease Pelvic sidewall compartment are free of disease Internal/external iliac arterial /venous branches are free of disease Sacrotuberous/sacrospinous Piriformis/Obturator Infralevator compartment Levator muscles are free of disease Sphincter complex are free of disease Anterior urogenital triangle/Perineum Vaginal introitus/urethra : free of disease Retropubic space: : free of disease Summary: MRI Overall stage: T N M , [ EMVI positive] [EMVI negative], [PSW positive ] [PSW negative], Total number of compartments, Closest potential surgical margins are located, Resection would require:
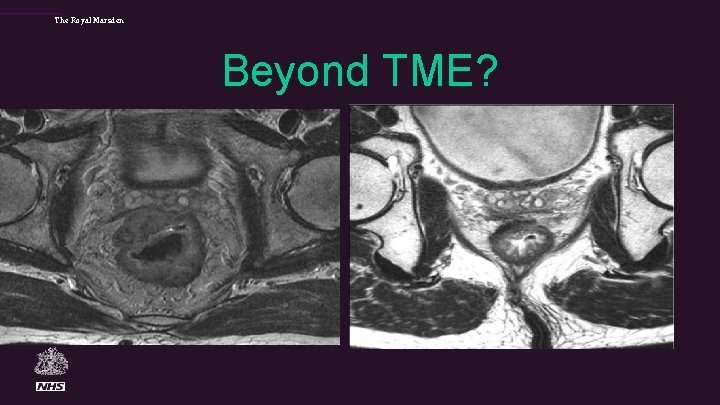
The Royal Marsden Beyond TME?
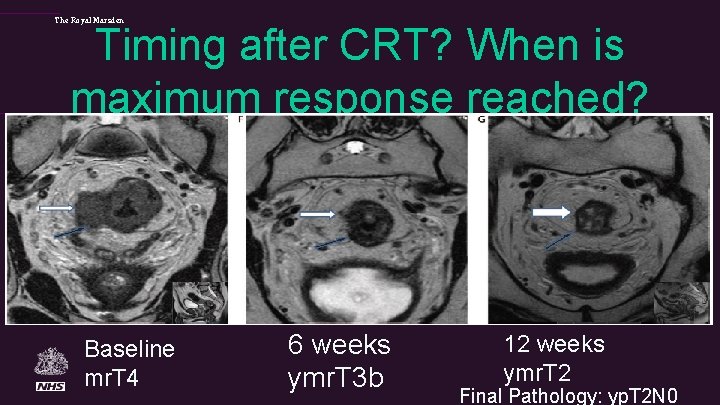
The Royal Marsden Timing after CRT? When is maximum response reached? Baseline mr. T 4 6 weeks ymr. T 3 b 12 weeks ymr. T 2 Final Pathology: yp. T 2 N 0
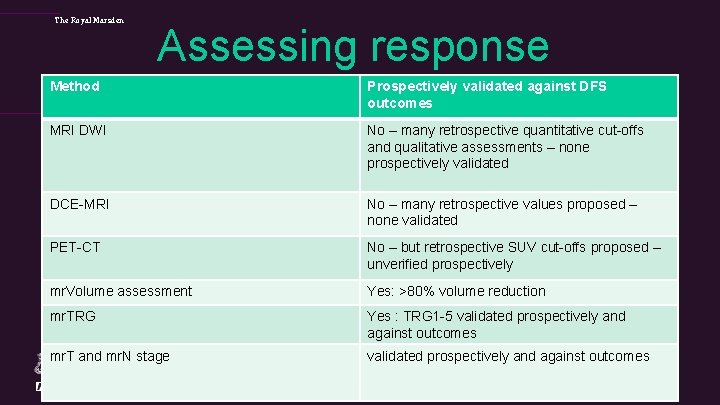
The Royal Marsden Assessing response Method Prospectively validated against DFS outcomes MRI DWI No – many retrospective quantitative cut-offs and qualitative assessments – none prospectively validated DCE-MRI No – many retrospective values proposed – none validated PET-CT No – but retrospective SUV cut-offs proposed – unverified prospectively mr. Volume assessment Yes: >80% volume reduction mr. TRG Yes : TRG 1 -5 validated prospectively and against outcomes mr. T and mr. N stage validated prospectively and against outcomes
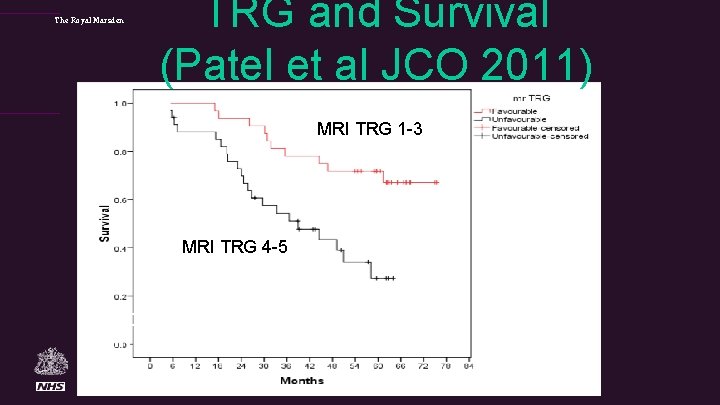
The Royal Marsden TRG and Survival (Patel et al JCO 2011) MRI TRG 1 -3 72% at 5 yrs MRI TRG 4 -5 27% at 5 yrs p=0. 001 HR 3. 28 (95%CI; 1. 22– 8. 80).
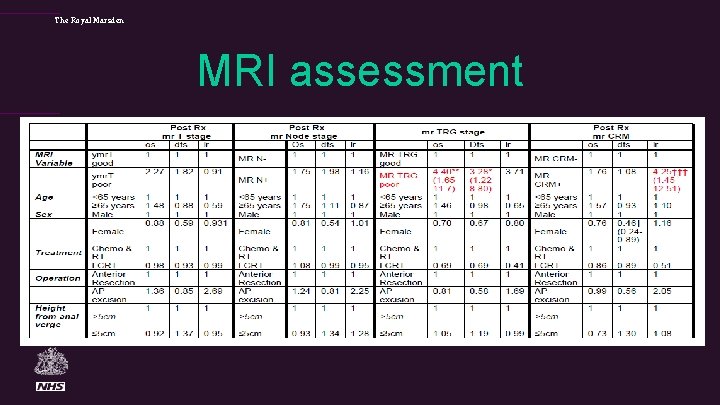
The Royal Marsden MRI assessment
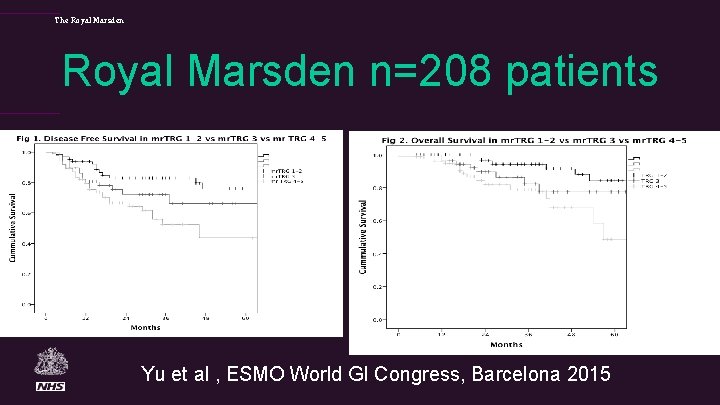
The Royal Marsden n=208 patients Yu et al , ESMO World GI Congress, Barcelona 2015
gina. brown@rmh. nhs. uk

The Royal Marsden SELECTING PATIENTS FOR DEFERRAL/WATCH AND WAIT POLICY
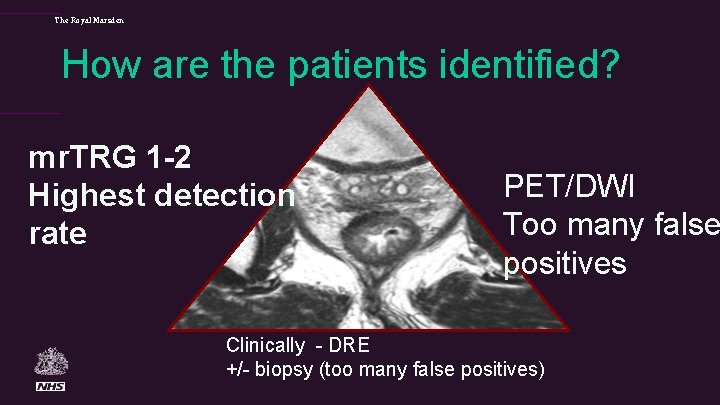
The Royal Marsden How are the patients identified? mr. TRG 1 -2 Highest detection rate PET/DWI Too many false positives Clinically - DRE +/- biopsy (too many false positives)
The Royal Marsden
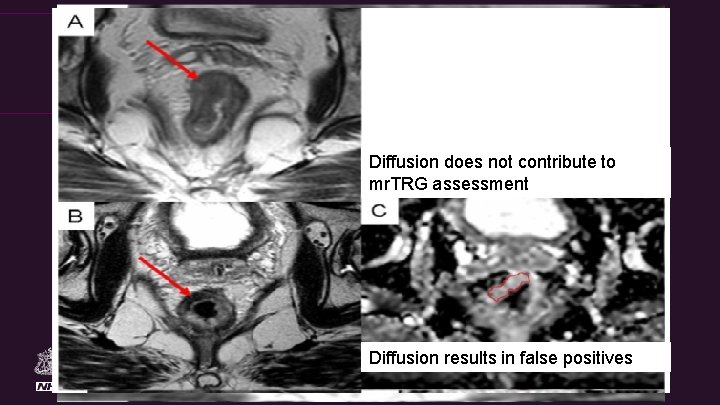
The Royal Marsden Diffusion does not contribute to mr. TRG assessment Diffusion results in false positives
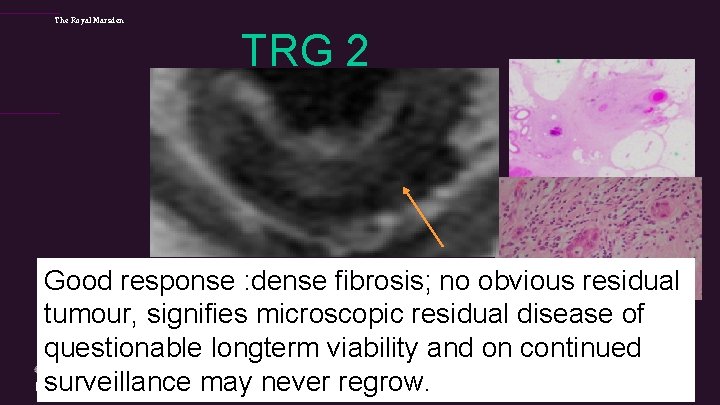
The Royal Marsden TRG 2 Good response : dense fibrosis; no obvious residual tumour, signifies microscopic residual disease of questionable longterm viability and on continued surveillance may never regrow.
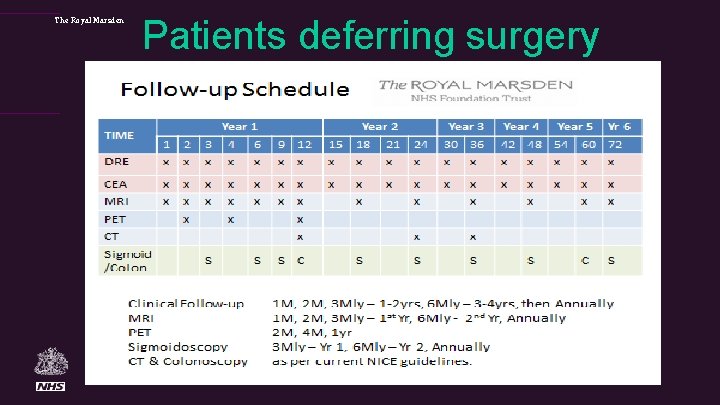
The Royal Marsden Patients deferring surgery
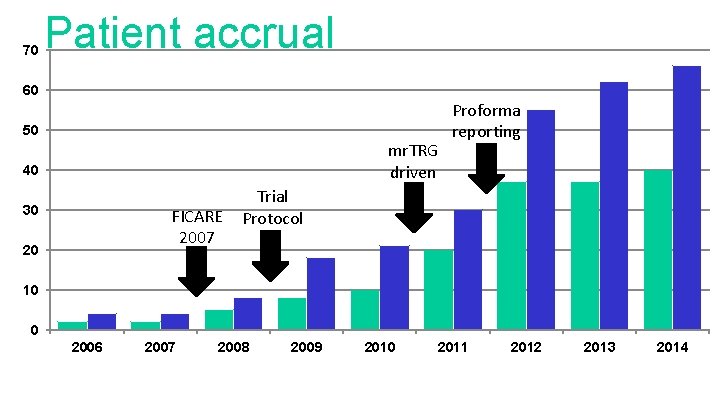
70 Patient accrual 60 50 mr. TRG driven 40 30 FICARE 2007 20 Proforma reporting Trial Protocol 10 0 2006 2007 2008 2009 2010 2011 2012 2013 2014

The Royal Marsden Proforma reporting
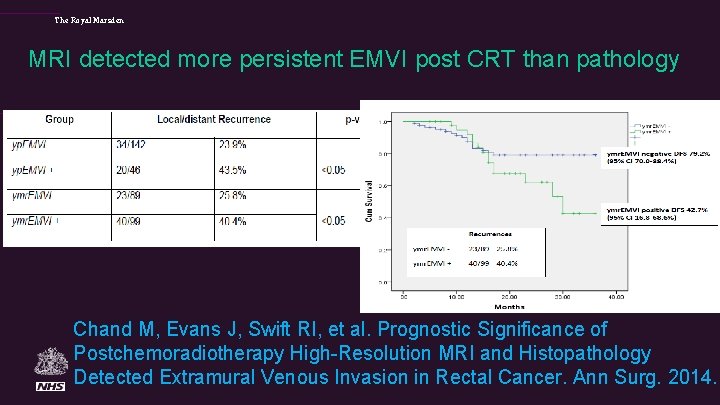
The Royal Marsden MRI detected more persistent EMVI post CRT than pathology Chand M, Evans J, Swift RI, et al. Prognostic Significance of Postchemoradiotherapy High-Resolution MRI and Histopathology Detected Extramural Venous Invasion in Rectal Cancer. Ann Surg. 2014.
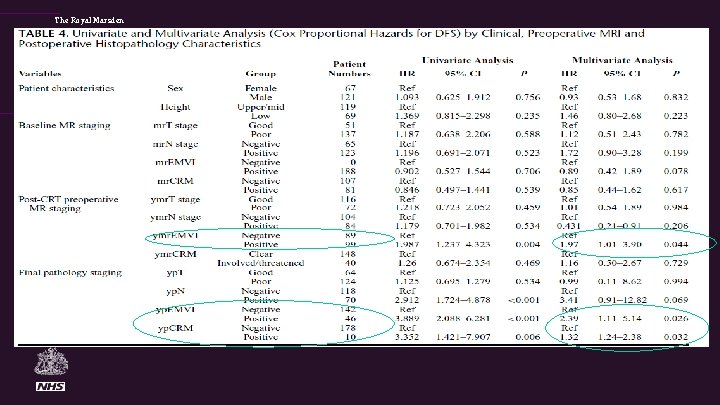
The Royal Marsden
The Royal Marsden • Shows good interobserver radiology agreement and reproducibility l l l • • mr. TRG is a prognostic (and predictive) biomarker MERCURY trial (JCO 2011 – multiple radiologists) EXPERT-C trial GEMCAD study (17 radiologists) CORE study (interobserver agreement) MERCURY 2 trial – risk factor for CRM involvement In EXPERT C trial identified 40% of patients with mr. TRG 1/2 – 89. 8% overall survival. Compared with only 15% pathologic CR rate (90% survival). Therefore mr. TRG could be justified as a more clinically relevant endpoint
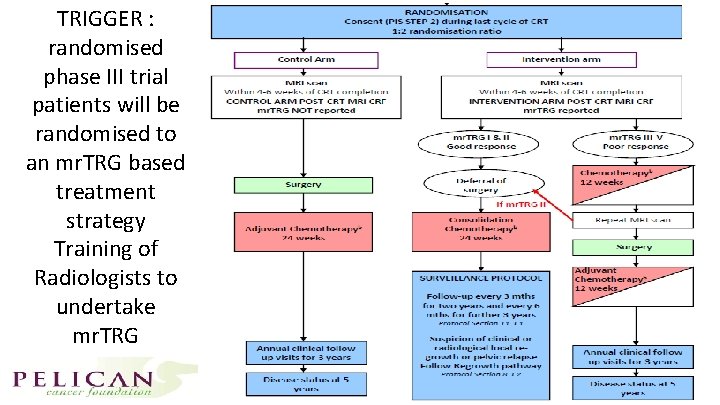
TRIGGER : randomised phase III trial patients will be randomised to an mr. TRG based treatment strategy Training of Radiologists to undertake mr. TRG
The Royal Marsden MRI reassessment after CRT • Philosophy of avoiding APE surgery if patient has had a good response to treatment • mr. TRG 1 -3 - used to identify patients suitable for deferral (many are falsely positive on biopsy, DWI and PET-CT) • Serial imaging – decision for deferral is not based on a single scan – uses the advantage of high resolution MRI monitoring • Employing serial MRI monitoring - gives opportunity to delay surgery until there is evidence of tumour regrowth rather than biopsy of tumour cells which are of uncertain viability
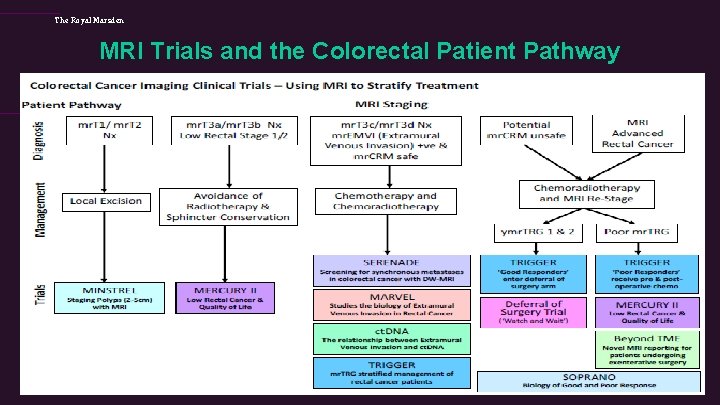
The Royal Marsden MRI Trials and the Colorectal Patient Pathway

The Royal Marsden 4 TH ANNUAL RECTAL MRI WORKSHOP FOR RADIOLOGISTS, SURGONS AND ONCOLOGISTS – 12 TH 13 TH JAN 2017

The Royal Marsden Faculty Discussants 2016 Prof John Nicholls Prof Paris Tekkis Prof Bill Heald Chris Tan Cunningham Arulampalam Roles: provoke discussion, share surgical and clinical experiences, highlight controversies, Summarise Key Messages

2016 Surgical, Oncology and Radiology participants The Royal Marsden GREENLAND ALASKA (USA) SWEDEN ICELAND RUSSIAN FEDERATION FINLAND NORWAY CANADA ESTONIA LATVIA DENMARK LITHUANIA BELARUS GERMANY POLAND REPULIC OF UNITED IRELAND KINGDOMNETHERLANDS BELGIUM CZECH REPUBLIC SLOVAKIA SWITZ. FRANCE UKRAINE KAZAKHSTAN AUSTRIA HUNGARY UZBEKISTAN BULGARIA UNITED STATES of AMERICA GEORGIA KYRGYZSTAN SPAIN NORTH KOREA GREECE TURKEY TURKMENISTAN SYRIA IRAQ TUNISIA MOROCCO IRAN LIBYA EGYPT SAUDI ARABIA MEXICO MAURITANIA MALI NIGER CHAD SENEGAL NICARAGUA GUINEA COSTA RICA PANAMA VENEZUELA GUYANA COLOMBIA FRENCH SURINAME GUIANA SUDAN GHANA TAIWAN VIETNAM MYANMAR LAOS THAILAND CAMBODIA NIGERIA ETHIOPIA CENTRAL AFRICAN REPUBLIC CAMEROON SOMALIA UGANDA PHILIPPINES SRI LANKA MALAYSIA INDONESIA PAPUA NEW GUINEA BRAZIL ANGOLA UE BIQ M ZIMBABWE NAMIBIA BOTSWANA MADAGASCAR O BOLIVIA ZAMBIA ZA PERU INDIA BURKINA COTE LIBERIAD’IVOIRE JAPAN NEPAL YEMEN KENYA CONGO GABON DEMOCRATIC REPUBLIC OF CONGO TANZANIA ECUADOR UAE OMAN SOUTH KOREA CHINA PAKISTAN WESTERN SAHARA CUBA TAHKISTAN AFGHANISTAN ALGERIA GUATEMALA HONDURAS MONGOLIA ROMANIA ITALY M number 2 1 61 3 1 2 1 1 1 7 2 2 1 3 7 1 1 PORTUGAL Country Australia Denmark England Germany Greece Italy Japan Lithuania Netherlands Norway Poland Portugal Russia Scotland Sweden USA Wales PARAGUAY AUSTRALIA CHILE URUGUAY REPUBLIC OF SOUTH AFRICA ARGENTINA NEW ZEALAND

Reporting Minimum Standards

- Slides: 39