CLASSICAL REACTIONs overview continued 2 Acidbase Complete Molecular
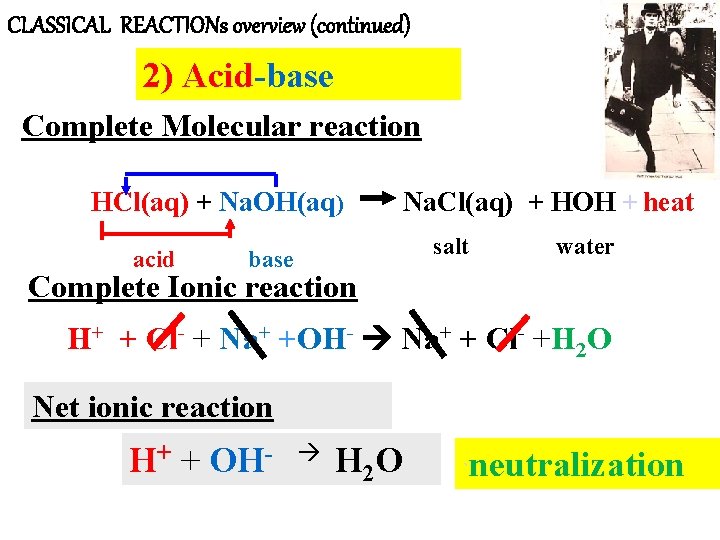
CLASSICAL REACTIONs overview (continued) 2) Acid-base Complete Molecular reaction HCl(aq) + Na. OH(aq) acid Na. Cl(aq) + HOH + heat salt base water Complete Ionic reaction H+ + Cl- + Na+ +OH- Na+ + Cl- +H 2 O Net ionic reaction H+ + OH- H 2 O ` neutralization
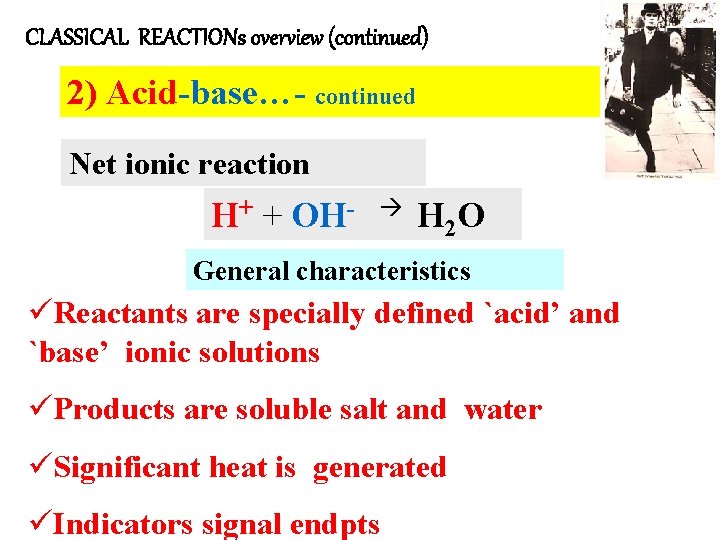
CLASSICAL REACTIONs overview (continued) 2) Acid-base…- continued Net ionic reaction H+ + OH- H 2 O General characteristics üReactants are specially defined `acid’ and `base’ ionic solutions üProducts are soluble salt and water üSignificant heat is generated üIndicators signal endpts
CLASSICAL REACTIONs overview (continued) 2) Acid-base…- continued Sometimes, Acid-Base reactions generate gas (g): HCl(aq) + Na(HCO 3)(aq) H 2 CO 3(aq) + HOH(=H 2 O) Gas-forming reaction follows formation of H 2 CO 3: H 2 CO 3(aq) H 2 O + CO 2(g)
CLASSICAL REACTIONs overview (continued) Acid-Base Theories (pp 163 -170, 652 -655) 0) Pre-science: acids and bases are eternal opposites ACID + BASE = `BALANCE’ Positive + negative = null Hot Taoist version + cold = just right

CLASSICAL REACTIONs overview (continued) Acid-Base Theories (pp 653 -656) Modern Theory: Try #1 Svante Arrhenius: Father of the first modern acid/base theory Thesis on Acids & Bases derided by his research committee… Graduates with Ph. D ordinare (no distinction) see p. 144 Young Arrhenius (not considered promising) Old Arrhenius (wins Nobel prize in 1903 for same acid base theory)

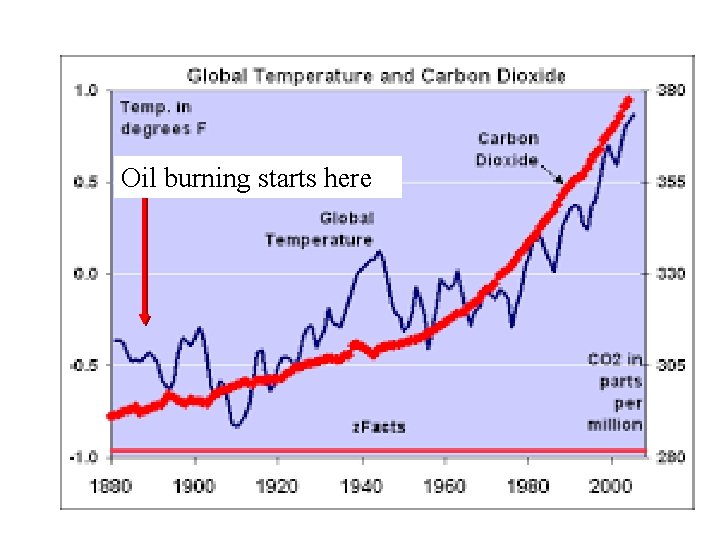
CLASSICAL REACTIONs OVERVIEW(continued) A measure of this Ph. D `ordinaire’’s brilliance… In 1896 Arrhenius predicted green house gas (CO 2) from profligate burning of the newly popular fuel source …petroleum oil… would cause (gasp !) measurable and catastrophic global warming… …starting in 1990 -2000 AD …the entire scientific establishment (and Standard Oil) laughed at him (again)

FYI…. Not a single current Republican member of the US Congress has yet to admit that global warming is caused by burning of fossil fuels.
Oil burning starts here
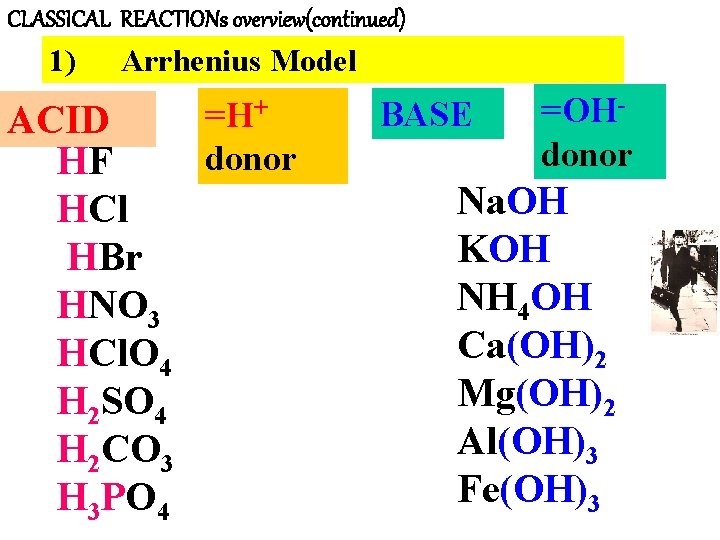
CLASSICAL REACTIONs overview(continued) 1) Arrhenius Model =H+ ACID donor HF HCl HBr HNO 3 HCl. O 4 H 2 SO 4 H 2 CO 3 H 3 PO 4 BASE =OHdonor Na. OH KOH NH 4 OH Ca(OH)2 Mg(OH)2 Al(OH)3 Fe(OH)3
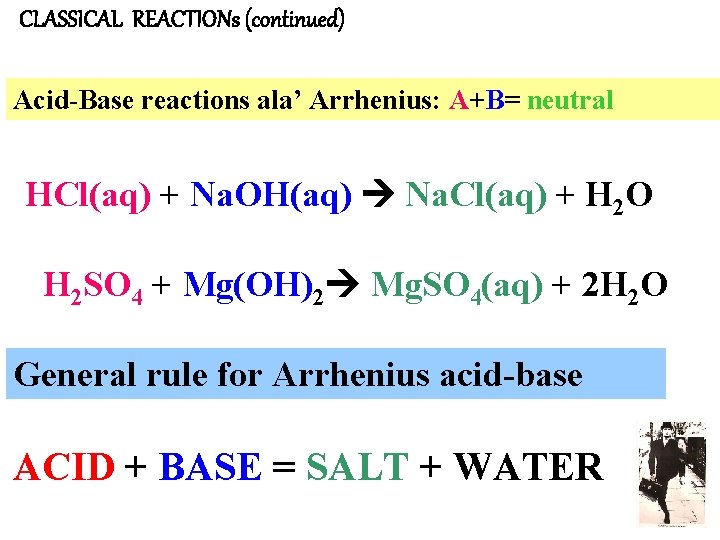
CLASSICAL REACTIONs (continued) Acid-Base reactions ala’ Arrhenius: A+B= neutral HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O H 2 SO 4 + Mg(OH)2 Mg. SO 4(aq) + 2 H 2 O General rule for Arrhenius acid-base ACID + BASE = SALT + WATER
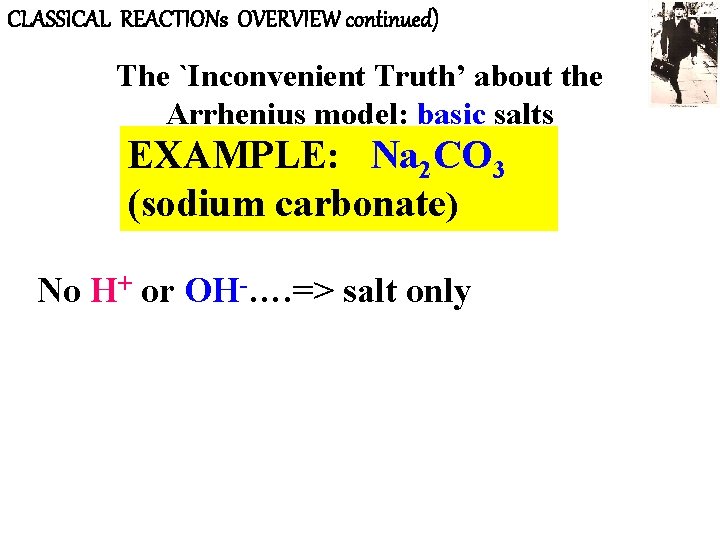
CLASSICAL REACTIONs OVERVIEW continued) The `Inconvenient Truth’ about the Arrhenius model: basic salts EXAMPLE: Na 2 CO 3 (sodium carbonate) No H+ or OH-…. => salt only

CLASSICAL REACTIONs OVERVIEW(continued) The `Inconvenient Truth’ about the Arrhenius model: basic salts (continued) EXAMPLE: Na 2 CO 3 (sodium carbonate) experimental results of adding to water: • Turns pink in presence of phenolphthalein • gas-forming reaction with HCl, pink disappears => A base !!!!? ? ? Where’s OH ? ? ?
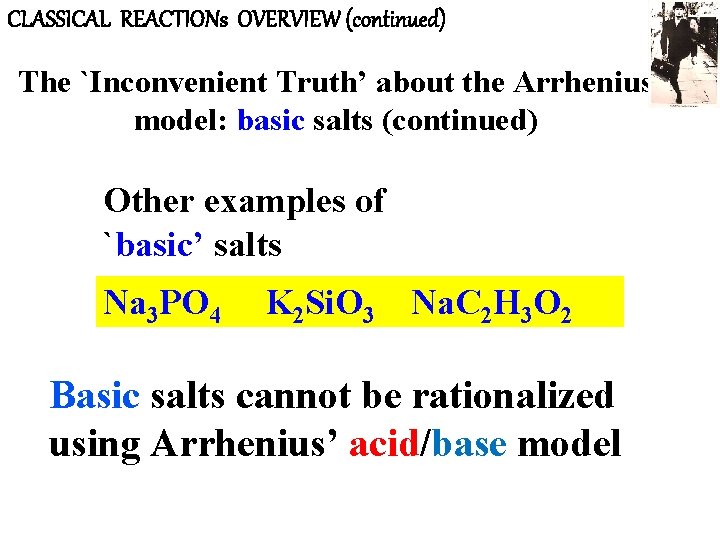
CLASSICAL REACTIONs OVERVIEW (continued) The `Inconvenient Truth’ about the Arrhenius model: basic salts (continued) Other examples of `basic’ salts Na 3 PO 4 K 2 Si. O 3 Na. C 2 H 3 O 2 Basic salts cannot be rationalized using Arrhenius’ acid/base model

CLASSICAL REACTIONs (continued) Bronsted. Winner to the rescue… of the Bronsted look alike contest…. Young Bronsted…Swedis h chemist circa 1910… Young James Dean…American actor circa 1955…(“Rebel Without a Cause, ”“East of Eden”, “Giant” ) Bronsted a few years after marriage and kids
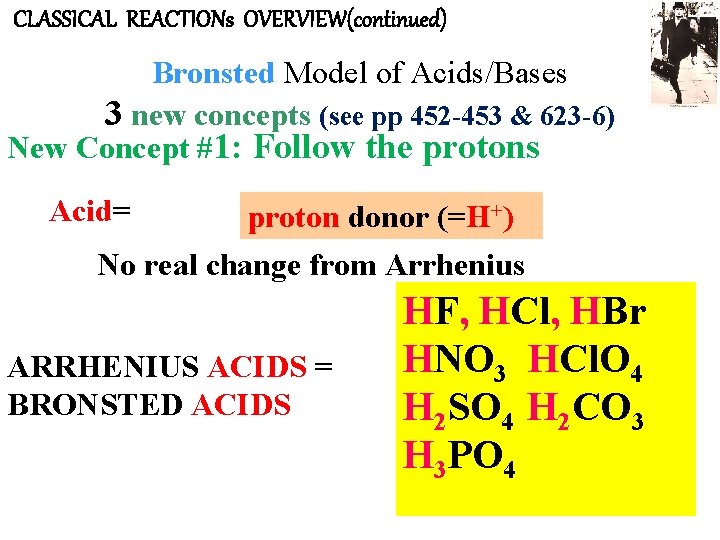
CLASSICAL REACTIONs OVERVIEW(continued) Bronsted Model of Acids/Bases 3 new concepts (see pp 452 -453 & 623 -6) New Concept #1: Follow the protons Acid= proton donor (=H+) No real change from Arrhenius ARRHENIUS ACIDS = BRONSTED ACIDS HF, HCl, HBr HNO 3 HCl. O 4 H 2 SO 4 H 2 CO 3 H 3 PO 4
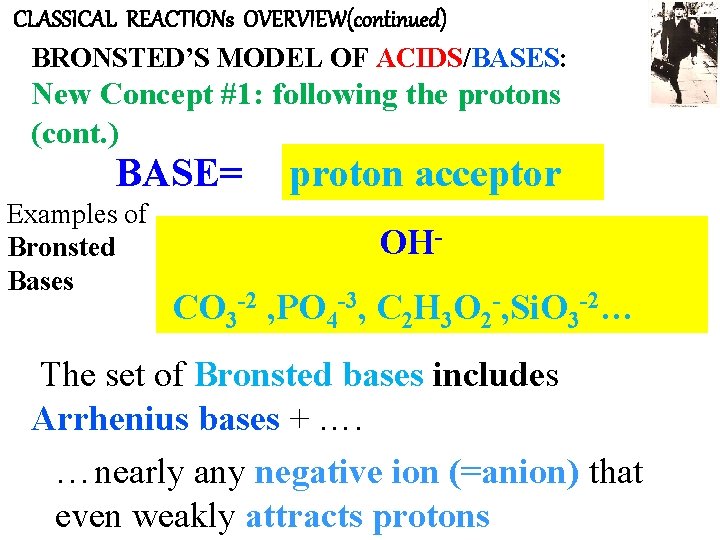
CLASSICAL REACTIONs OVERVIEW(continued) BRONSTED’S MODEL OF ACIDS/BASES: New Concept #1: following the protons (cont. ) BASE= Examples of Bronsted Bases proton acceptor OH- CO 3 -2 , PO 4 -3, C 2 H 3 O 2 -, Si. O 3 -2… The set of Bronsted bases includes Arrhenius bases + …. …nearly any negative ion (=anion) that even weakly attracts protons
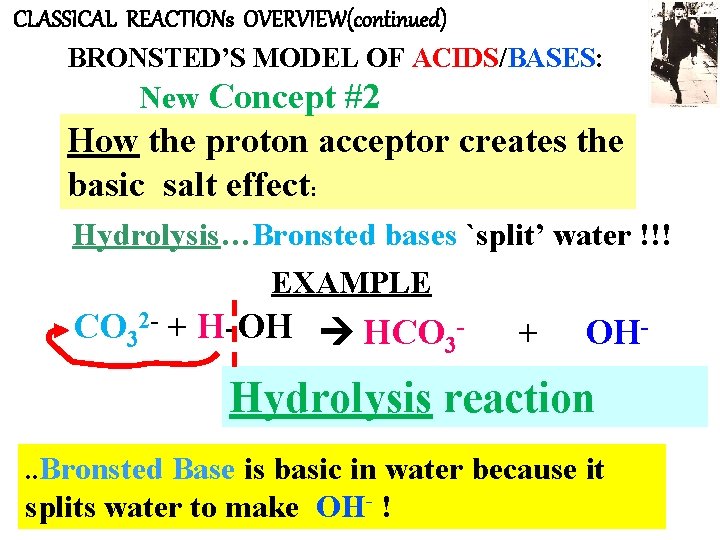
CLASSICAL REACTIONs OVERVIEW(continued) BRONSTED’S MODEL OF ACIDS/BASES: New Concept #2 How the proton acceptor creates the basic salt effect: Hydrolysis…Bronsted bases `split’ water !!! EXAMPLE CO 32 - + H-OH HCO 3 - + OH- Hydrolysis reaction. . Bronsted Base is basic in water because it splits water to make OH- !
- Slides: 17