Class Notes Naming and Writing Formulas 2 ways


















- Slides: 22

Class Notes: Naming and Writing Formulas

• 2 ways to determine the charge on an ion: 1. By group in the P. T. : Group number tells number of valence electrons, which determines whether atom needs to gain or lose electrons

2. By Name: used for most transition metals and two representative metals by including a Roman numeral in the name Ex. Roman Numerals – Iron(II) = Fe 2+ – Iron(III) = Fe 3+ – Copper(I) = Cu+ – Copper(II) = Cu 2+ • Memorize exceptions: Ag+, Zn 2+, Cd 2+; Sn, Pb

Binary Ionic Compounds made of 2 parts -1 cation and 1 anion = metal & nonmetal *Writing the formula from the name (+) must equal (-) -write the formulas for both ions -put the cation (positive ion) first -show the charges

*The criss-cross (crossing-over) method Switch the charges for the ions and write them as subscripts Calcium bromide = Ca. Br 2 Iron (III) oxide = Fe 2 O 3

*subscripts must be in the lowest whole number ratio Calcium sulfide = Ca. S *writing the name from the formula -metal + nonmetal-”ide” Ca. Cl 2 = Calcium chloride K 2 S = Potassium sulfide

Ionic Compounds with Polyatomics

• Polyatomic Ions = a group of atoms which behave as a unit and carry a charge • Be able to recognize polyatomics • -all anions except ammonium NH 4+ • -all but 3 end in –ite or –ate (exceptions: CN- cyanide, OHhydroxide, ammonium)

Ionic Compounds with Polyatomics -contains atoms of 3 or more different elements -contain 1 or more polyatomic ions

*Writing the formulas from the name -same as for a binary ionic -treat the polyatomic as ONE ion -write the formulas for the two ions showing charges - criss-cross charges

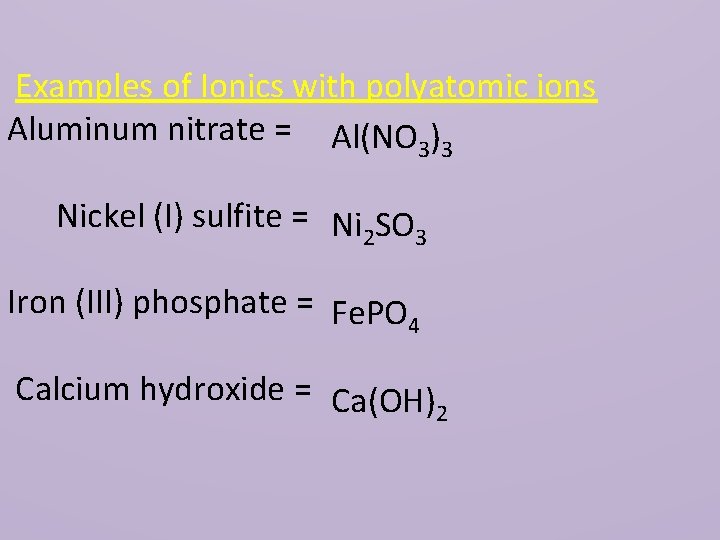
Examples of Ionics with polyatomic ions Aluminum nitrate = Al(NO 3)3 Nickel (I) sulfite = Ni SO 2 3 Iron (III) phosphate = Fe. PO 4 Calcium hydroxide = Ca(OH) 2

Writing the name from the formula -name the cation first -Write the name of the polyatomic Mg 3(PO 4)2 = Magnesium Phosphate Na. CN = Sodium Cyanide NH 4 NO 2 = Ammonium Nitrite NH 4 Cl = Ammonium Chloride

Binary Molecular Compounds

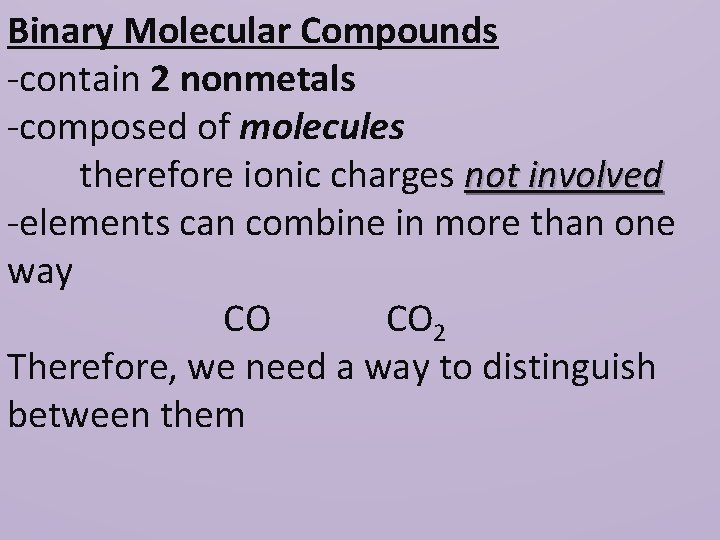
Binary Molecular Compounds -contain 2 nonmetals -composed of molecules therefore ionic charges not involved -elements can combine in more than one way CO CO 2 Therefore, we need a way to distinguish between them

Prefixes = use to show numbers of each atom in the molecule (never used with metal/ionic compounds) -all binary compounds end in –”ide” *memorize: mono = 1 6 di =2 tri =3 tetra = 4 hexa = hepta = 7 octa = 8 nona = 9

Naming -be on the lookout for 2 nonmetals -subscripts are the prefixes -don’t put mono- in front of 1 st element -drop first vowel if two vowels together, except (i)

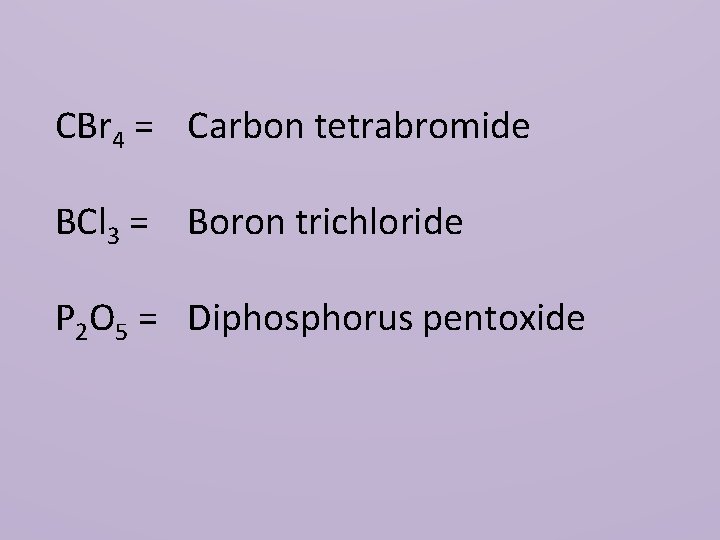
CBr 4 = Carbon tetrabromide BCl 3 = Boron trichloride P 2 O 5 = Diphosphorus pentoxide

Formulas – prefixes are the subscripts Carbon dioxide = Carbon monoxide = CO 2 CO Dinitrogen pentasulfide = N 2 S 5 Triphosphorous hexabromide = P 3 Br 6

Acids -compounds which give H+ ions when dissolved in water Generic Formula: HA = H+ and A- (a negative anion or polyatomic ion)

3 Rules for Naming Acids – they depend on the anion name 1. when the anion name ends in –ide, put “hydro-“ in front; “-ic” on the end, “acid” after HCl Cl- = chloride hydrochloric acid H 2 S S- = sulfide hydrosulfuric acid

2. when the anion ends in –ite, put “-ous” on the end, “acid” after H 2 SO 3 HNO 2 SO 32 - = sulfite sulfurous acid NO 21 - = nitrite nitrous acid

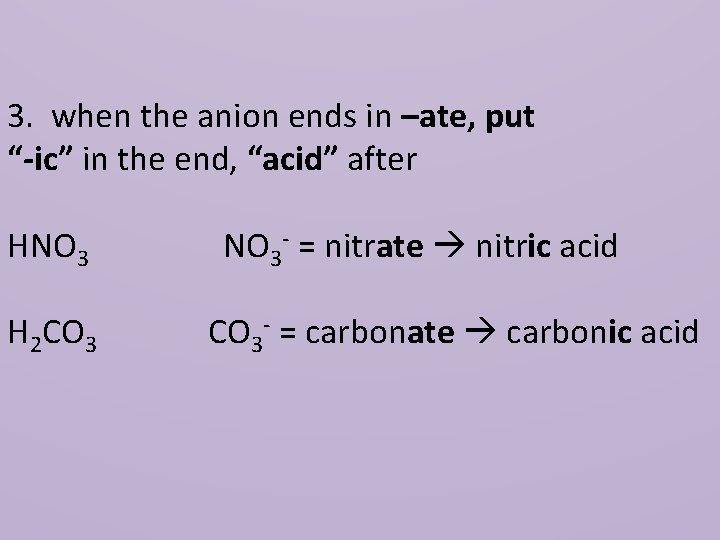
3. when the anion ends in –ate, put “-ic” in the end, “acid” after HNO 3 H 2 CO 3 NO 3 - = nitrate nitric acid CO 3 - = carbonate carbonic acid