Class content Structures chaps 1 4 Properties chaps




















- Slides: 41






Class content • • • Structures (chaps. 1 -4) Properties (chaps. 5 -8) Phase (chaps. 9 -10) Materials type (chaps. 11 -16) Performance (chaps. 17 -23)

LECTURES Lecturer: Prof. Jacob C. Huang Rm 4006, Tel: 4063, Jacobc@mail. nsysu. edu. tw Time: Monday, 9 -12 am Activities: • Present new materials • Announce reading and homework • Direct lab tour, lab report • Take quizzes, midterms, and final

TEACHING ASSISTANTS Name Office 謝中翰 B 5017 蔡濰猷 B 5017 Tel. 525 -4070 E-mail a 123321833@yahoo. com. tw 0963 -469 -803 m 013100033@student. nsysu. edu. tw 0921 -790 -857 Teaching Assistants will • participate in recitation sessions, • have office hours to help you with course material and problem sets.

COURSE WEBSITES Course Website: NSYSU Web Univ • Syllabus • Lecture notes • Mail list • Message Text Website: http: //www. wiley. com/college/callister • Additional Chapters (Chapters 19 -23) • Complete solutions to selected problems • Links to other web resources • Extended learning objectives • Self-assessment exercises

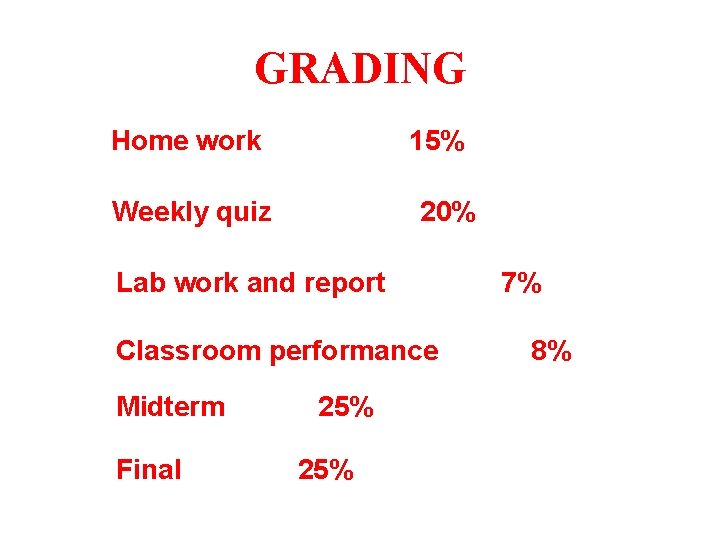
GRADING Home work 15% Weekly quiz 20% Lab work and report Classroom performance Midterm Final 25% 7% 8%

Chapter 1 Introduction

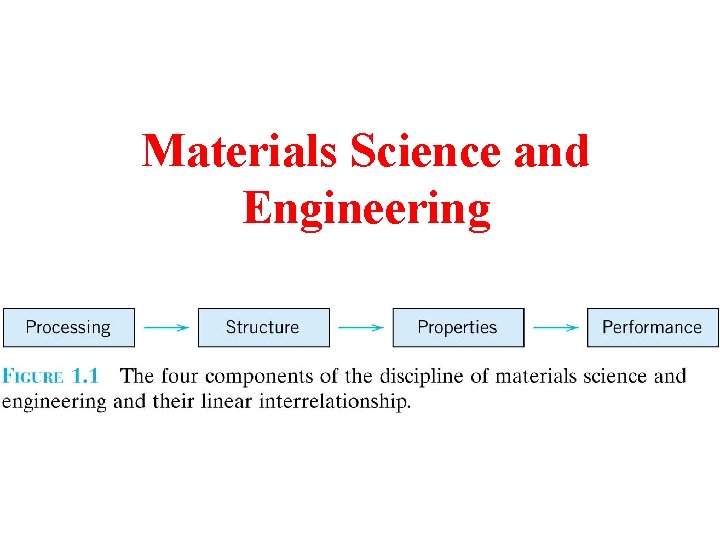
Materials Science and Engineering

Structure, Processing, & Properties • Properties depend on structure ex: hardness vs structure of steel (d) Hardness (BHN) 600 500 400 (c) (a) (b) 4 mm 300 200 30 mm 100 0. 01 0. 1 30 mm Data obtained from Figs. 10. 30(a) and 10. 32 with 4 wt% C composition, and from Fig. 11. 14 and associated discussion, Callister 7 e. Micrographs adapted from (a) Fig. 10. 19; (b) Fig. 9. 30; (c) Fig. 10. 33; and (d) Fig. 10. 21, Callister 7 e. 1 10 1000 Cooling Rate (ºC/s) • Processing can change structure ex: structure vs cooling rate of steel

• Materials drive our society –Stone Age –Bronze Age –Iron Age –Now? • Silicon Age? • Polymer Age?

Materials study • Materials design • Materials selection • Materials degradation • Materials cost





Materials classifications • Metals • Ceramics • Polymers • • • Composites Semiconductors Biomaterials Smart materials Nano materials

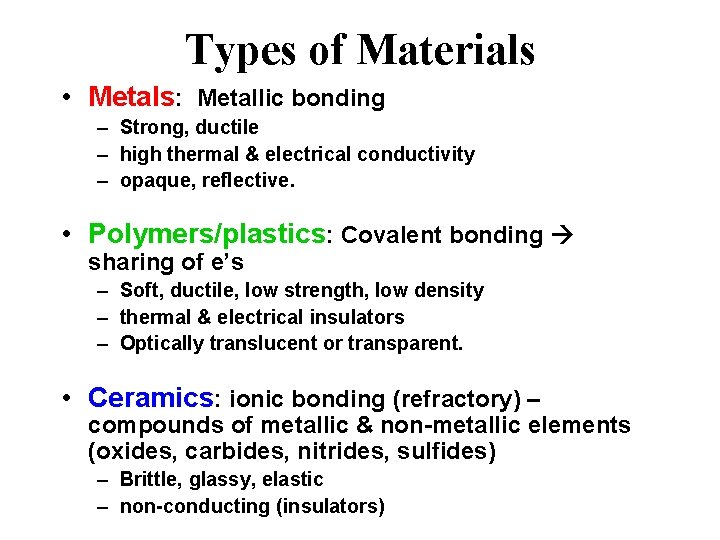
Types of Materials • Metals: Metallic bonding – Strong, ductile – high thermal & electrical conductivity – opaque, reflective. • Polymers/plastics: Covalent bonding sharing of e’s – Soft, ductile, low strength, low density – thermal & electrical insulators – Optically translucent or transparent. • Ceramics: ionic bonding (refractory) – compounds of metallic & non-metallic elements (oxides, carbides, nitrides, sulfides) – Brittle, glassy, elastic – non-conducting (insulators)

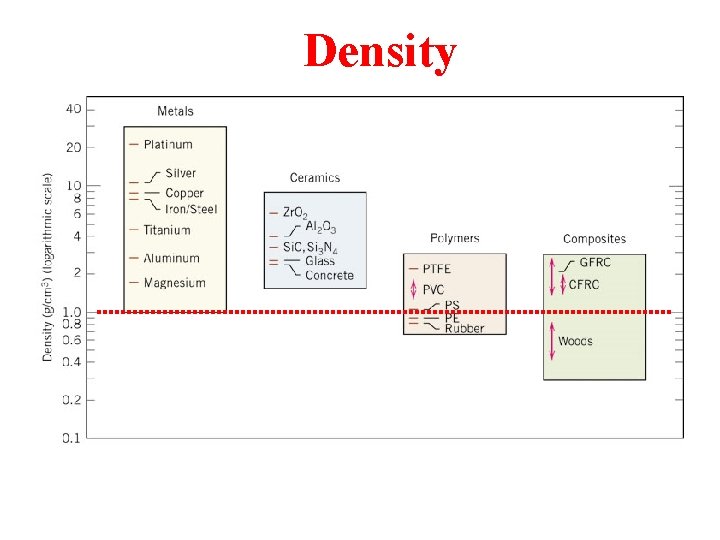
Density

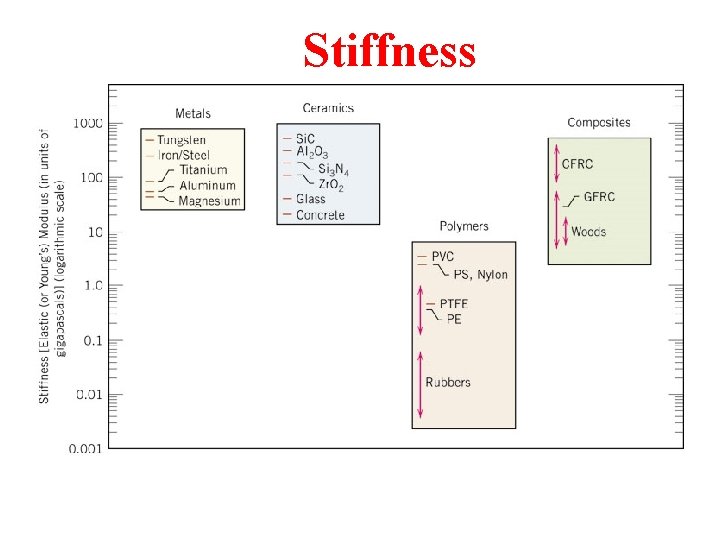
Stiffness

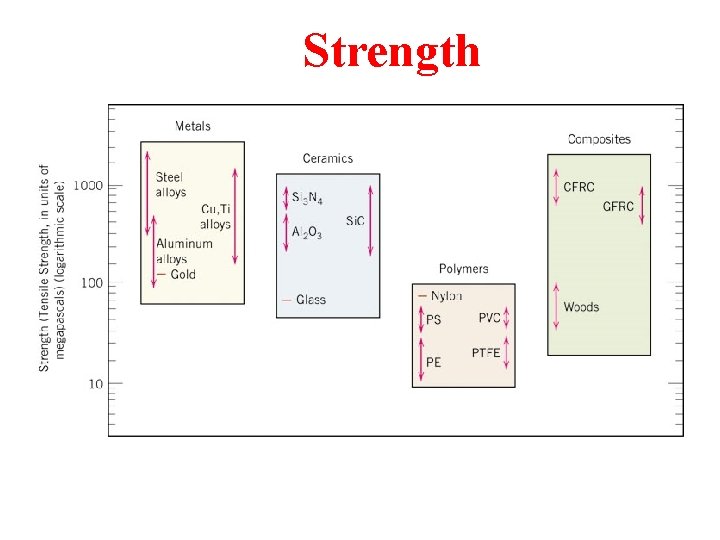
Strength

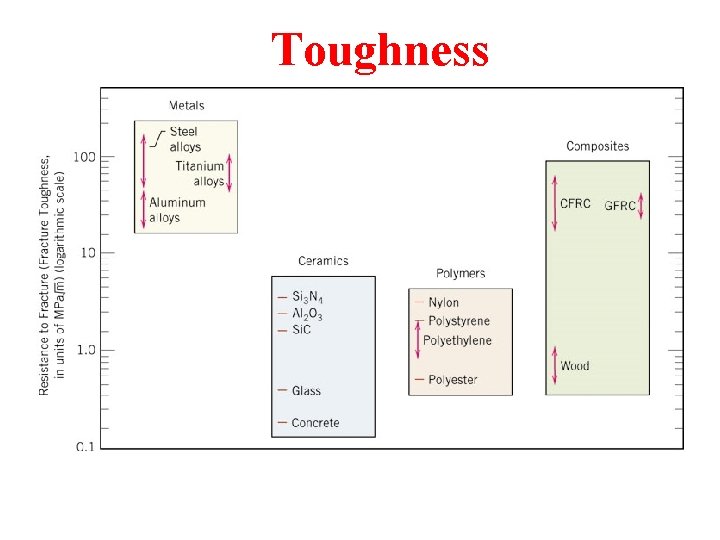
Toughness

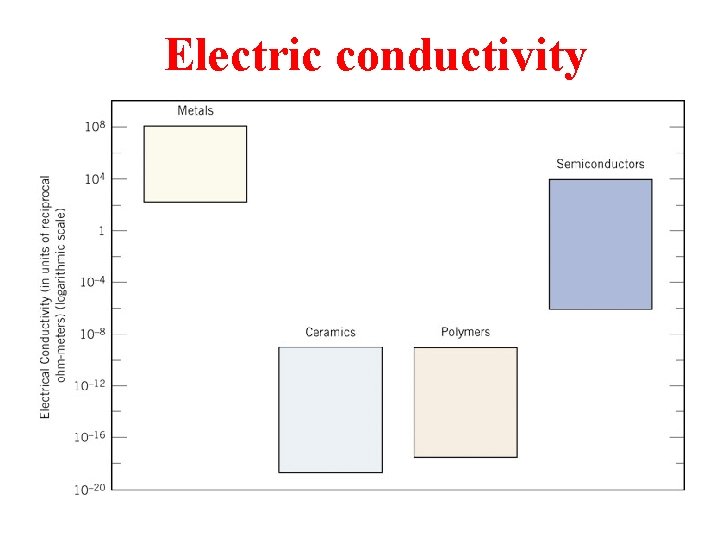
Electric conductivity

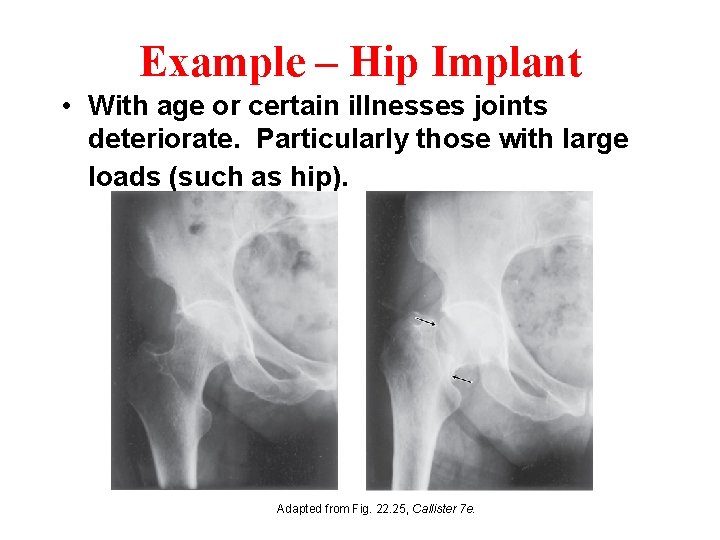
Example – Hip Implant • With age or certain illnesses joints deteriorate. Particularly those with large loads (such as hip). Adapted from Fig. 22. 25, Callister 7 e.

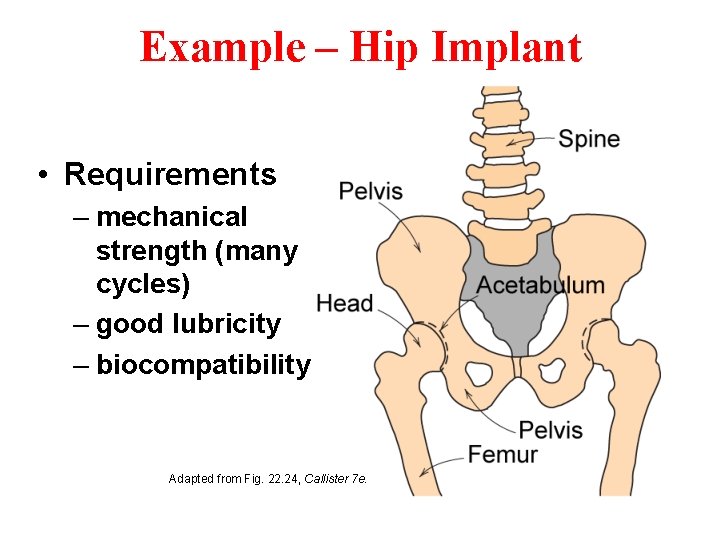
Example – Hip Implant • Requirements – mechanical strength (many cycles) – good lubricity – biocompatibility Adapted from Fig. 22. 24, Callister 7 e.

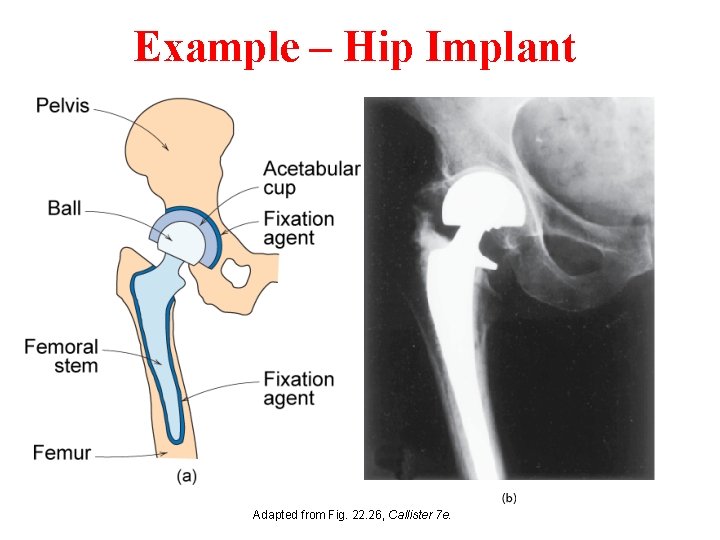
Example – Hip Implant Adapted from Fig. 22. 26, Callister 7 e.

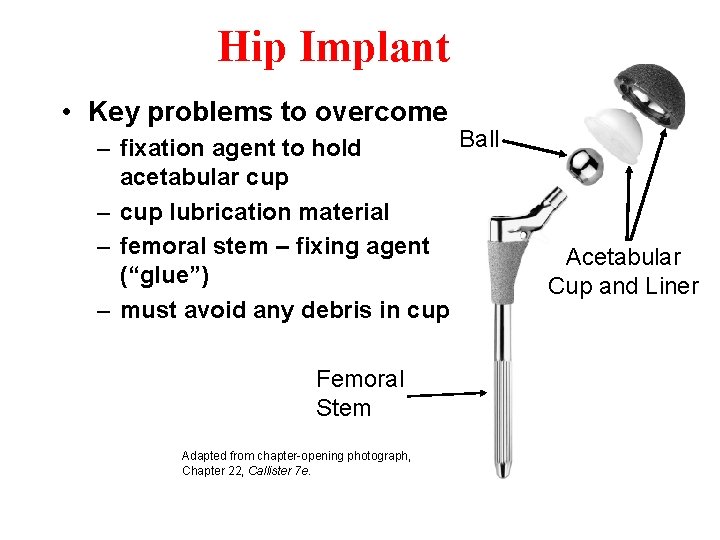
Hip Implant • Key problems to overcome Ball – fixation agent to hold acetabular cup – cup lubrication material – femoral stem – fixing agent (“glue”) – must avoid any debris in cup Femoral Stem Adapted from chapter-opening photograph, Chapter 22, Callister 7 e. Acetabular Cup and Liner

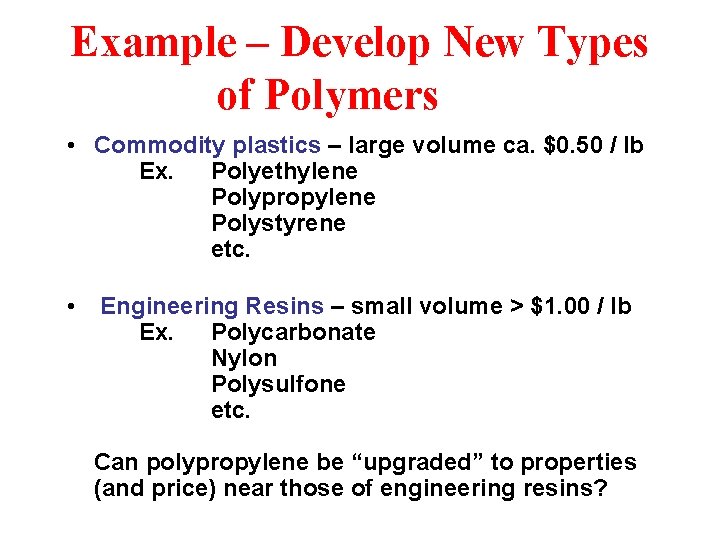
Example – Develop New Types of Polymers • Commodity plastics – large volume ca. $0. 50 / lb Ex. Polyethylene Polypropylene Polystyrene etc. • Engineering Resins – small volume > $1. 00 / lb Ex. Polycarbonate Nylon Polysulfone etc. Can polypropylene be “upgraded” to properties (and price) near those of engineering resins?

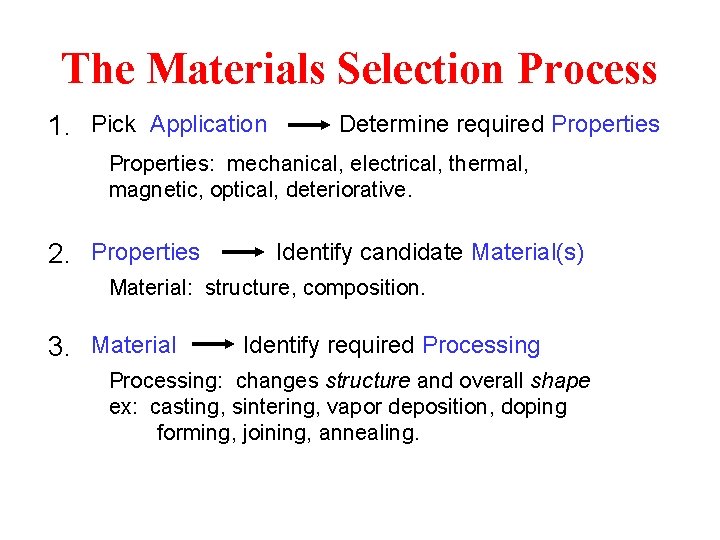
The Materials Selection Process 1. Pick Application Determine required Properties: mechanical, electrical, thermal, magnetic, optical, deteriorative. 2. Properties Identify candidate Material(s) Material: structure, composition. 3. Material Identify required Processing: changes structure and overall shape ex: casting, sintering, vapor deposition, doping forming, joining, annealing.

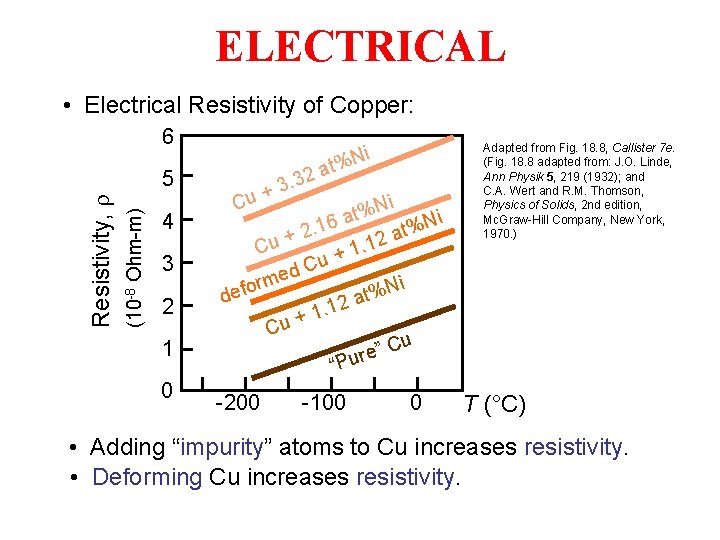
ELECTRICAL • Electrical Resistivity of Copper: 6 (10 -8 Ohm-m) Resistivity, r 5 4 3 2 1 0 Ni Cu + t% a 2 3. 3 Ni % t Ni 16 a. % t 2 a + 2 1. Cu +1 u C d e rm Ni % t defo 12 a. 1 + Cu Cu ” e r “Pu -200 -100 0 Adapted from Fig. 18. 8, Callister 7 e. (Fig. 18. 8 adapted from: J. O. Linde, Ann Physik 5, 219 (1932); and C. A. Wert and R. M. Thomson, Physics of Solids, 2 nd edition, Mc. Graw-Hill Company, New York, 1970. ) T (°C) • Adding “impurity” atoms to Cu increases resistivity. • Deforming Cu increases resistivity.

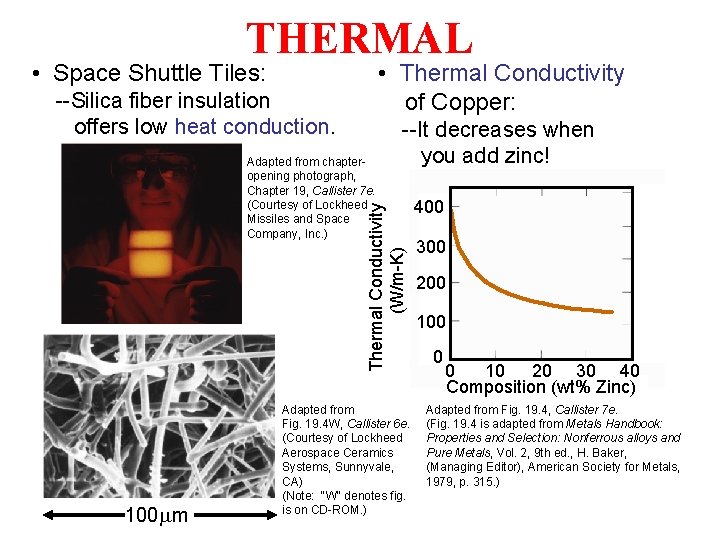
THERMAL • Space Shuttle Tiles: • Thermal Conductivity of Copper: --Silica fiber insulation offers low heat conduction. Thermal Conductivity (W/m-K) Adapted from chapteropening photograph, Chapter 19, Callister 7 e. (Courtesy of Lockheed Missiles and Space Company, Inc. ) --It decreases when you add zinc! 100 mm Adapted from Fig. 19. 4 W, Callister 6 e. (Courtesy of Lockheed Aerospace Ceramics Systems, Sunnyvale, CA) (Note: "W" denotes fig. is on CD-ROM. ) 400 300 200 100 0 0 10 20 30 40 Composition (wt% Zinc) Adapted from Fig. 19. 4, Callister 7 e. (Fig. 19. 4 is adapted from Metals Handbook: Properties and Selection: Nonferrous alloys and Pure Metals, Vol. 2, 9 th ed. , H. Baker, (Managing Editor), American Society for Metals, 1979, p. 315. )

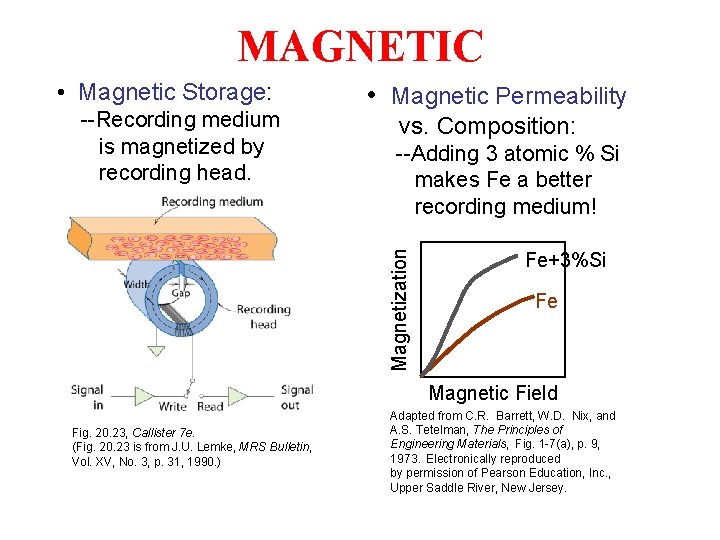
MAGNETIC • Magnetic Storage: vs. Composition: --Adding 3 atomic % Si makes Fe a better recording medium! Magnetization --Recording medium is magnetized by recording head. • Magnetic Permeability Fe+3%Si Fe Magnetic Field Fig. 20. 23, Callister 7 e. (Fig. 20. 23 is from J. U. Lemke, MRS Bulletin, Vol. XV, No. 3, p. 31, 1990. ) Adapted from C. R. Barrett, W. D. Nix, and A. S. Tetelman, The Principles of Engineering Materials, Fig. 1 -7(a), p. 9, 1973. Electronically reproduced by permission of Pearson Education, Inc. , Upper Saddle River, New Jersey.

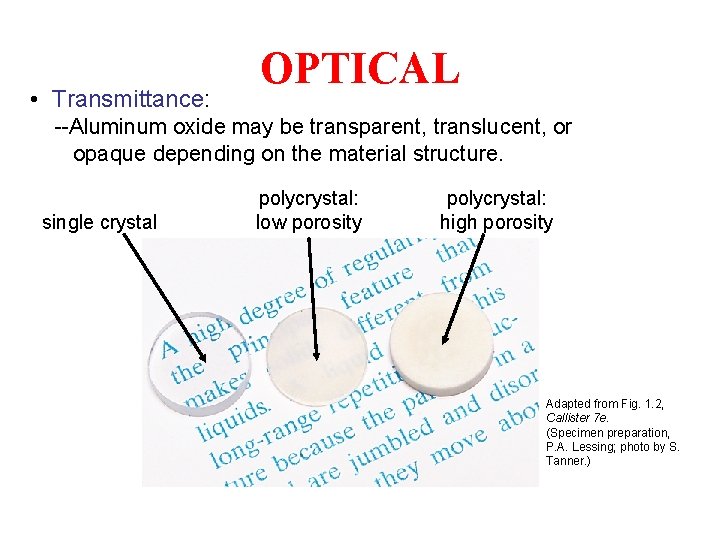
• Transmittance: OPTICAL --Aluminum oxide may be transparent, translucent, or opaque depending on the material structure. single crystal polycrystal: low porosity polycrystal: high porosity Adapted from Fig. 1. 2, Callister 7 e. (Specimen preparation, P. A. Lessing; photo by S. Tanner. )

f 02_01_pg 4

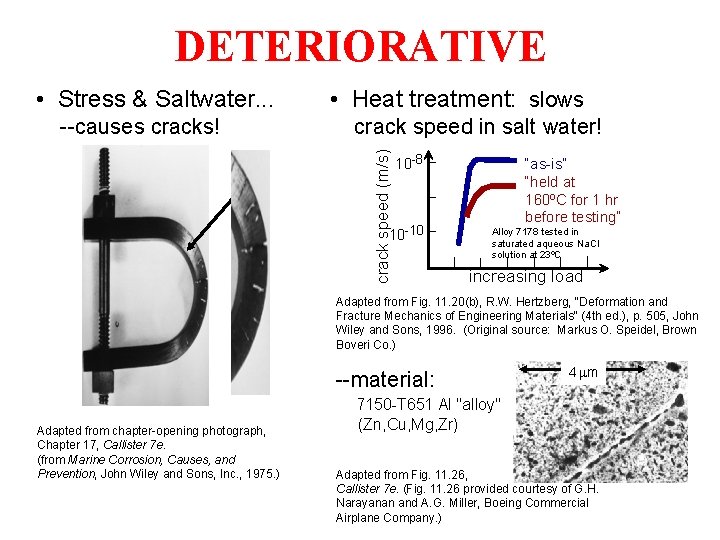
DETERIORATIVE • Stress & Saltwater. . . crack speed in salt water! crack speed (m/s) --causes cracks! • Heat treatment: slows 10 -8 10 -10 “as-is” “held at 160ºC for 1 hr before testing” Alloy 7178 tested in saturated aqueous Na. Cl solution at 23ºC increasing load Adapted from Fig. 11. 20(b), R. W. Hertzberg, "Deformation and Fracture Mechanics of Engineering Materials" (4 th ed. ), p. 505, John Wiley and Sons, 1996. (Original source: Markus O. Speidel, Brown Boveri Co. ) --material: Adapted from chapter-opening photograph, Chapter 17, Callister 7 e. (from Marine Corrosion, Causes, and Prevention, John Wiley and Sons, Inc. , 1975. ) 4 mm 7150 -T 651 Al "alloy" (Zn, Cu, Mg, Zr) Adapted from Fig. 11. 26, Callister 7 e. (Fig. 11. 26 provided courtesy of G. H. Narayanan and A. G. Miller, Boeing Commercial Airplane Company. )

SUMMARY Course Goals: • Use the right material for the job. • Understand the relation between properties, structure, and processing. • Recognize new design opportunities offered by materials selection.

Issues to note • SI Unit and unit conversion (Appd. A; final page) • English names of pure elements (p. 25; front and final pages) http: //www. chemicool. com/ http: //www. webelements. com/ • Selective properties of some important pure elements (Appd. B) • Cost (Appd. C) • Glossary or terminology (G 1 -G 15) • Index (I 1 -I 22) • References (end of each chapter)

SI units (A 1 -A 2) • SI base units: m, kg, s, K, A, mol • SI deviated units • SI multiples: T, G, M, k, m, m, n, p