Class 6 Phase Diagrams And Microstructure Homeworks If



- Slides: 12

Class 6 Phase Diagrams And Microstructure

Homeworks • If everyone agrees, homeworks will be in the lab on a bench in alphabetical groups. • Placed there Wednesday so can be collected after 1. 00 pm • Lab schedule starts Wed 2. 00 pm room 121 Crawford Hall. • NOTE First Exam move to Oct 10 th • Multiple Choice – eg how does sodium and chlorine bond?

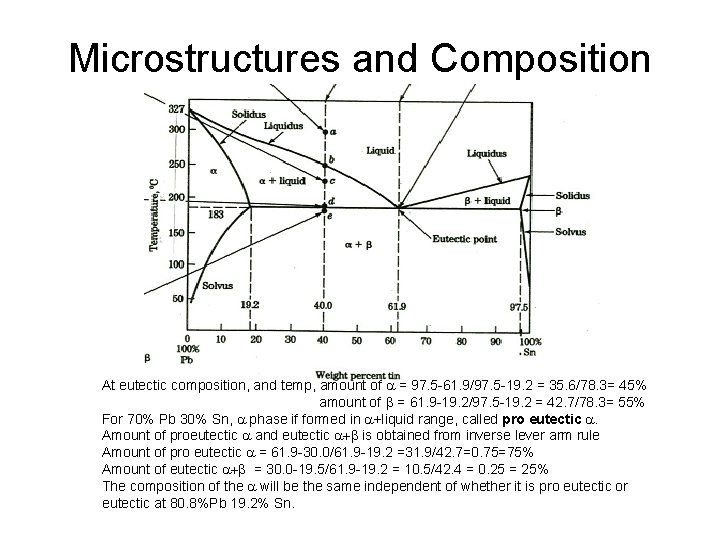
Microstructures and Composition At eutectic composition, and temp, amount of a = 97. 5 61. 9/97. 5 19. 2 = 35. 6/78. 3= 45% amount of b = 61. 9 19. 2/97. 5 19. 2 = 42. 7/78. 3= 55% For 70% Pb 30% Sn, a phase if formed in a+liquid range, called pro eutectic a. Amount of proeutectic a and eutectic a+b is obtained from inverse lever arm rule Amount of pro eutectic a = 61. 9 30. 0/61. 9 19. 2 =31. 9/42. 7=0. 75=75% Amount of eutectic a+b = 30. 0 19. 5/61. 9 19. 2 = 10. 5/42. 4 = 0. 25 = 25% The composition of the a will be the same independent of whether it is pro eutectic or eutectic at 80. 8%Pb 19. 2% Sn.

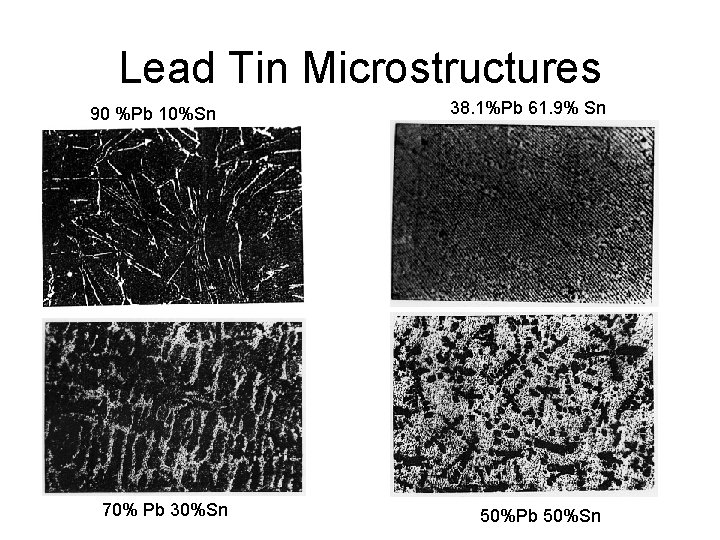
Lead Tin Microstructures 90 %Pb 10%Sn 70% Pb 30%Sn 38. 1%Pb 61. 9% Sn 50%Pb 50%Sn

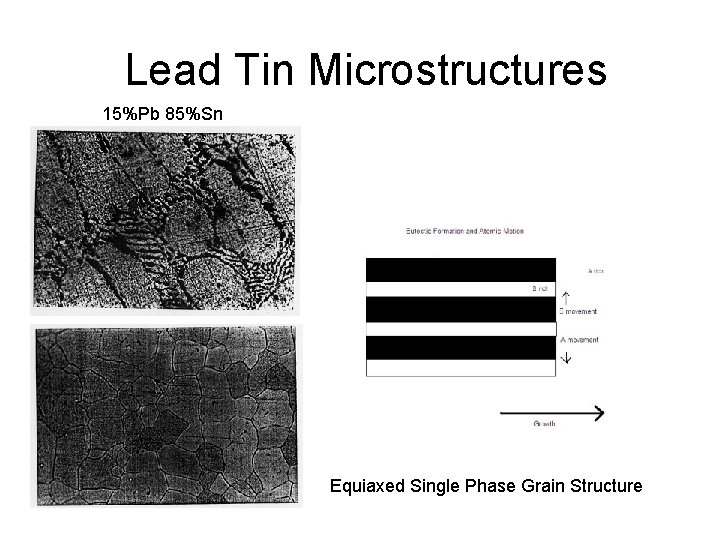
Lead Tin Microstructures 15%Pb 85%Sn Equiaxed Single Phase Grain Structure

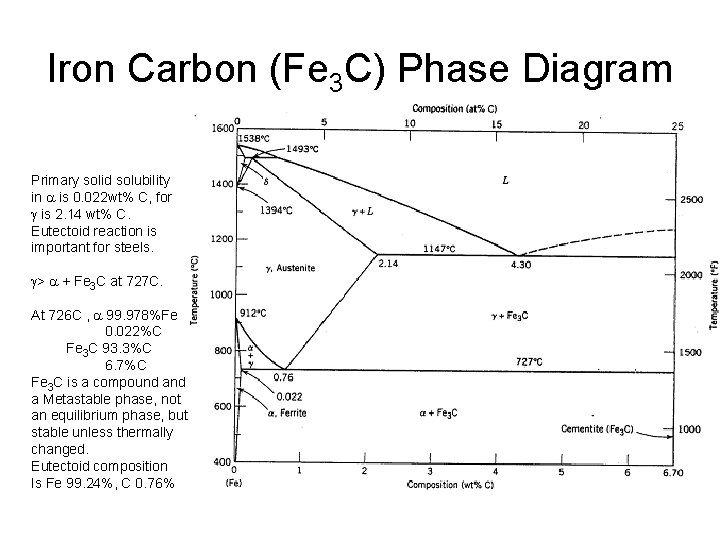
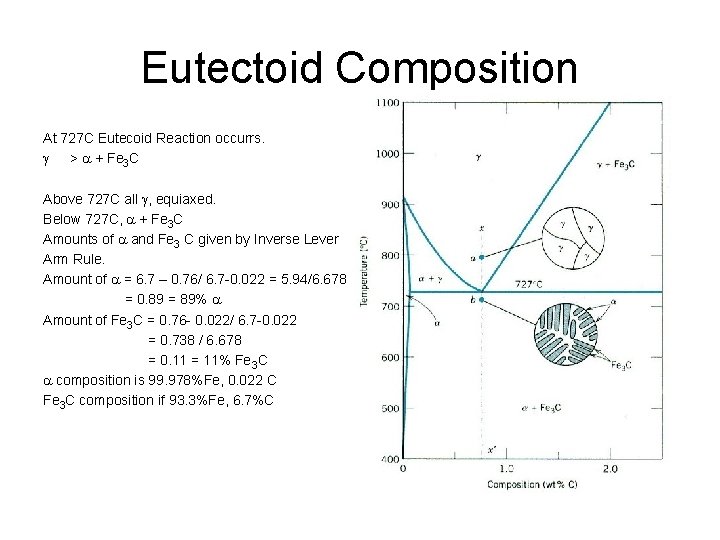
Iron Carbon (Fe 3 C) Phase Diagram Primary solid solubility in a is 0. 022 wt% C, for g is 2. 14 wt% C. Eutectoid reaction is important for steels. g> a + Fe 3 C at 727 C. At 726 C , a 99. 978%Fe 0. 022%C Fe 3 C 93. 3%C 6. 7%C Fe 3 C is a compound a Metastable phase, not an equilibrium phase, but stable unless thermally changed. Eutectoid composition Is Fe 99. 24%, C 0. 76%

Eutectoid Composition At 727 C Eutecoid Reaction occurrs. g > a + Fe 3 C Above 727 C all g, equiaxed. Below 727 C, a + Fe 3 C Amounts of a and Fe 3 C given by Inverse Lever Arm Rule. Amount of a = 6. 7 – 0. 76/ 6. 7 0. 022 = 5. 94/6. 678 = 0. 89 = 89% a Amount of Fe 3 C = 0. 76 0. 022/ 6. 7 0. 022 = 0. 738 / 6. 678 = 0. 11 = 11% Fe 3 C a composition is 99. 978%Fe, 0. 022 C Fe 3 C composition if 93. 3%Fe, 6. 7%C

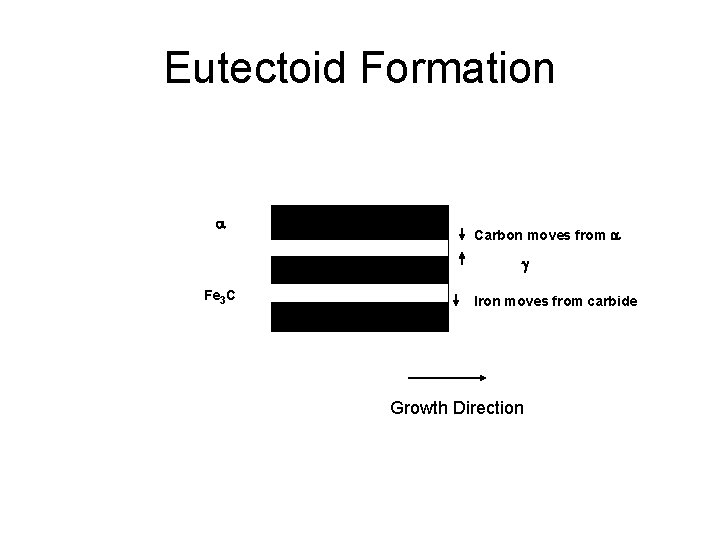
Eutectoid Formation a Carbon moves from a g Fe 3 C Iron moves from carbide Growth Direction

Eutectoid Alternate ferrite and cementite plate structure in Eutectoid steel.

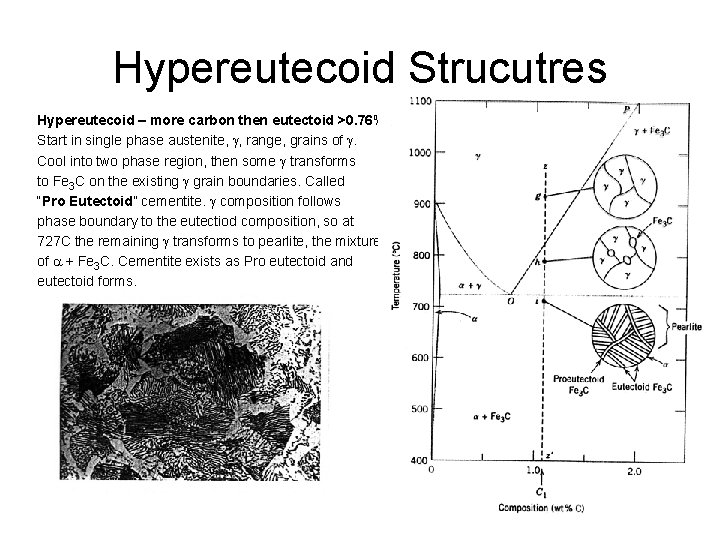
Hypereutecoid Strucutres Hypereutecoid – more carbon then eutectoid >0. 76% Start in single phase austenite, g, range, grains of g. Cool into two phase region, then some g transforms to Fe 3 C on the existing g grain boundaries. Called “Pro Eutectoid” cementite. g composition follows phase boundary to the eutectiod composition, so at 727 C the remaining g transforms to pearlite, the mixture of a + Fe 3 C. Cementite exists as Pro eutectoid and eutectoid forms.

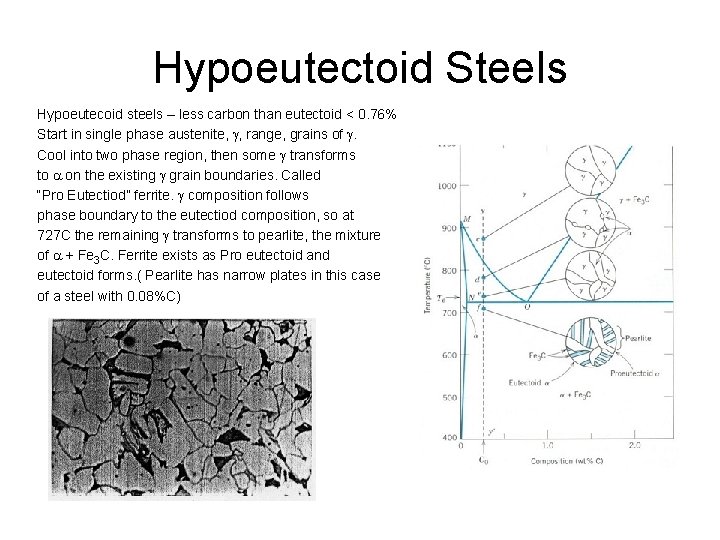
Hypoeutectoid Steels Hypoeutecoid steels – less carbon than eutectoid < 0. 76% Start in single phase austenite, g, range, grains of g. Cool into two phase region, then some g transforms to a on the existing g grain boundaries. Called “Pro Eutectiod” ferrite. g composition follows phase boundary to the eutectiod composition, so at 727 C the remaining g transforms to pearlite, the mixture of a + Fe 3 C. Ferrite exists as Pro eutectoid and eutectoid forms. ( Pearlite has narrow plates in this case of a steel with 0. 08%C)

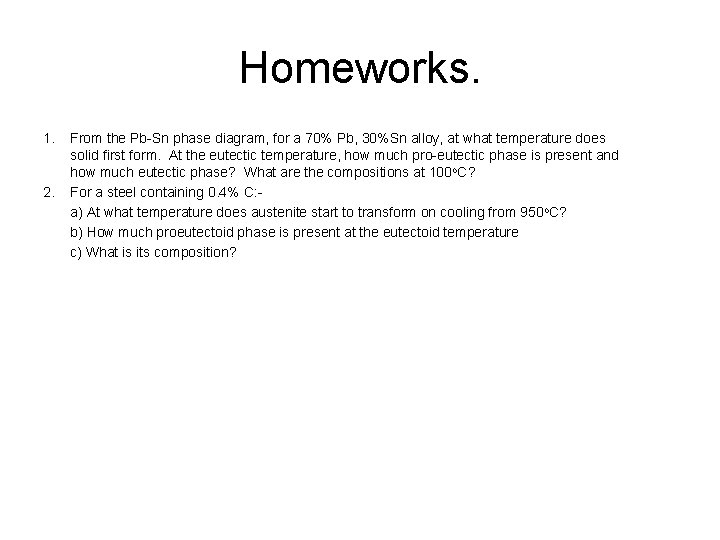
Homeworks. 1. 2. From the Pb Sn phase diagram, for a 70% Pb, 30%Sn alloy, at what temperature does solid first form. At the eutectic temperature, how much pro eutectic phase is present and how much eutectic phase? What are the compositions at 100 o. C? For a steel containing 0. 4% C: a) At what temperature does austenite start to transform on cooling from 950 o. C? b) How much proeutectoid phase is present at the eutectoid temperature c) What is its composition?