Citric acid cycle Definition The citric acid cycle










- Slides: 30

Citric acid cycle

Definition The citric acid cycle is the final common pathway for the oxidation of fuel molecules such as amino acids, fatty acids, and carbohydrates. Most fuel molecules enter the cycle as acetyl coenzyme A. The reactions of the citric acid cycle take place inside mitochondria, in contrast with those of glycolysis, that take place in the cytosol. Under aerobic conditions, the pyruvate generated from glucose is oxidatively decarboxylated to form acetyl Co. A.

Overview of the citric acid cycle Citric acid cycle also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle. The cycle is also an important source of precursors, not only for the storage forms of fuels, but also for the building blocks of many other molecules such as amino acids, nucleotide bases, cholesterol, and porphyrin (the organic component of heme) What is the function of TCA cycle in transforming fuel molecules into ATP?

Remember that fuel molecules are carbon compounds that are capable of being oxidized of losing electrons. The citric acid cycle includes a series of oxidation reduction reactions that result in the oxidation of an acetyl group to 2 molecules of carbon dioxide. A four carbon compound (oxaloacetate) condenses with a two carbon acetyl unit to yield a six carbn tricarboxylic acid (citrate). An isomer of citrate is then oxidatively decarboxylated. The resulting five carbon compound (α ketoglutarate) also is oxidatively decaroxylated to yield a four carbon compound (succinate). Oxaloacetate is then regenerated from succinate

Two carbon atoms enter the cycle as an acetyl unit and two carbon atoms lave the cycle in the form of two molecules of carbon dioxide. Three hydride ions (so, six electrons) re transferred to three molecules of nicotinamide adenine dinucleotide (NAD+), whereas one pair of hydrogen atoms (therefore, 2 electrons) is transferred to one molecule of flavin adenine dinucleotide (FAD). The function of the citric acid cycle I the harvesting of high energy electrons from carbon fuels. NOTE: that TCA cycle itself neither generates a large amount of ATP nor includes oxygen as a reactant.

1 The citric acid cycle begins with the condensation of a four carbon unit, oxaloacetate, and a two carbon unit, the acetyl group of acetyl Co. A. Oxaloacetate reacts with acetyl Co. A and H 2 O to yield citrate (C 6), which isomerized to isocitrate (C 6). 2 Oxidative decarboxylation of this intermeddiate gives α ketoglutarate (C 5). 3 The second molecule of carbon dioxide comes off in the next reaction, in which α ketoglutarate is oxidatively decarboxylated to succinyl Co. A(C 4).

4 The thioester bond of succinyl Co. A is cleaved by inorthophosphate to yield succinate, and a high phosphoryl transfer potential compound in the form of GTP is along with generated. 5 Succinate is oxidized to fumarate (C 4), which is then hydrated to form malate (C 4). 6 Finally, malate is oxidized to regenerate oxaloacetate (C 4).

Therefore, 2 carbon atoms from acetyl Co. A enter the cycle, and 2 carbon atoms leave the cycle as CO 2 in the successive decarboxylations catalyzed by isocitrate dehydrogenase and α ketoglutara Dehydrogenase. In the four oxidation reduction reactions in the cycle, 3 pairs of electrons are transferred to NAD+ and one pair to FAD. These reduced electrons carriers are subsequently oxidized by the electron transport chain to generate approximately 9 molecules of ATP.

In addition, 1 molecule of a compound having a high phosphoryl transfer potential is directly formed in the citric acid cycle. Hence, a total of 10 molecules of compounds having high phosphoryl transfer potential are generated for each two carbon fragment that is completely oxidized to H 2 O and CO 2.

The disruption of pyruvate metabolism is the cause Beriberi and poisoning by Mercury and Arsenic Beriberi, a neurologic and cardiovascular disorder, is caused by a dietary deficiency of thiamine (vitamin B 1). The disease is characterized by neurologic and cardiac symptoms. Damage to the peripheral nervous system is expressed as pain in the limbs, kness, and unclear skin sensation. Heart may enlarged and the cardiac output inadequate. In Beriberi the levels of pyruvate and α ketoglutarate in the blood are higher than normal.

The increase in the level of pyruvate in the blood is especially obvious After the ingestion of glucose. A related finding is that the activity of the pyruvate and α ketoglutarate dehydrogenase complexes in vivo are abnormally low. The low transketolase activity of red cells in Beriberi is an easily measured and reliable diagnostic indicator of the disease. The binding of mercury or arsenite to the dihydropoyl groups inhibits the complex and leads to central nervous system pathologies. Treatment for these poisons is the administration of 2, 3 dimercaptopropanol was developed after World War 1 as an antidote to lewisite, an arsenic based chemical weapon. This compound was initially called BAL, for British anti lewisite.

Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH 2 to O 2 by a series of electron carrier. This process take place in mitochondria. The flow of electrons from NADH or FADH 2 to O 2 through protein complexes located in the mitochondrial inner membrane leads to the pumping of protons out of the mitochondrial matrix. The resulting uneven distribution of protons generates a PH gradient and a transmembrane electrical potential that creates a proton motive force.

ATP is synthesized when protons flow back to the mitochondrial matrix through an enzyme complex. Therefore, the oxidation of fuels and the phosphorylation of ADP are coupled by a proton gradient across the inner mitochondrial membrane. - Oxidative phosphorylation is the culmination of a series of energy transformations that are called cellular respiration. 1 carbon fuels are oxidized in the citric acid cycle to yield electrons with high transfer potential. 2 This electron motive force is converted into a proton motive force.

3 finally, the proton motive force is converted into phosphoryl transfer potential. The conversion of electron motive force into proton motive force is carried out by 3 electron driven proton pumps NADH. Q oxidoreductase, Q cytochrome c oxidoreductase, and cytochrome c oxidase. These large transmembrane complexes contain multiple oxidation reduction centers, including quinones, flavins, iron sulfur clusters, hemes, and copper ions. The final phase of oxidative phosphorylation is carried out by ATP synthase, an ATP synthesizing assembly that is driven by the flow of protons back into the mitochondrial matrix.

Oxidative phosphorylation brightly shows that proton gradients are an interconvertible currency of free energy in biological systems. Toxic derivatives of molecular oxygen such as superoxide radical are scavenge by protective enzymes Although cytochrome c oxidase and other proteins that reduce O 2 are remarkably successful in not releasing intermediates, small amounts of superoxide anion and hydrogen peroxide are unavoidably formed. Superoxide, hydrogen peroxide, and species that can be generated from them such as OH· are collectively referred to as reactive oxygen species or ROS.

What are the cellular defense strategies against oxidative damage by ROS? The enzyme superoxide dismutase. This enzyme scavenges superoxide radicals by catalyzing the conversion of two of these radicals into hydrogen peroxide and molecular oxygen. 2 O 2· + 2 H+ ↔ O 2 + H 2 O 2 Eukaryotes contain 2 forms of this enzyme, manganese containing version located in mitochondria and copper zinc dependent cytosolic form. a) The oxidized form of the enzyme is reduced by superoxide to form O 2.

b) The reduced form of the enzyme, formed in this reaction then reacts with a second superoxide ion to form peroxide, which takes 2 protons along the reaction path to yield hydrogen peroxide. The hydrogen peroxide formed by superoxide dismutase and by other processes is scavenged by catalase, a ubiquitous heme protein that catalyzes the dismutation of hydrogen peroxide into water and O 2. 2 H 2 O 2 ↔ O 2 + 2 H 2 O Superoxde dismutase and catalase are remarkable efficient, performing their reactions at or near the diffusion limited rate. Other cellular defenses against oxidative damage include the antioxidant vitamins, vitamins E and C.

Because it is lipophilic, vitamin E is especially useful in protecting membranes from lipid peroxidation. The importance of the cell’s defense against ROS is demonstrated by the presence of superoxidee dismutase In all aerobic organisms. Oxidative damage is believed to cause, at least in part, a growing of diseases such as, Atherogenesis, Diabetes, parkinson disease, acute renal failure and cervical cancer

Coordinated control of ATP production Glycolysis, the citric acid cycle, and oxidative phosphorylation compose the major pathways for cellular ATP production. Control of oxidative phosphorylation by the ATP mass action ratio [ATP] / [ADP][Pi] depends, of course on an adequate supply of electrons to fuel the electron transport chain. This aspect of the system’s control is, in turn, dependent on the [NADH] / [NAD+] ratio, which is maintained high by the combined action of glycolysis and the citric acid cycle in converting 10 molecles of NAD+ to NADH per molecule of glucose oxidized. Therefore, that coordinated control is necessary for the three processes.

This is provided by the regulation of each of the control points of glycolysis [hexokinase, phosphofructokinase (PFK), and pyruvate kinase] and the citric acid cycle [ pyruvate dehydrogenase, citrate synthase, isocitrate dehydrogenase, and α ketoglutarate dehydrogenase] by adenine nuclotides or NADH or both as well as by certain metabolites. Citrate inhibits glycolysis The main control points of glycolysis and citric acid cycle are regulated by several effectors besides adenine nuclotides or NADH. One particularly interesting regulatory effect is the inhibition of PFK by citrate. When demand for ATP decreases, [ATP] increases and [ADP] decreases.

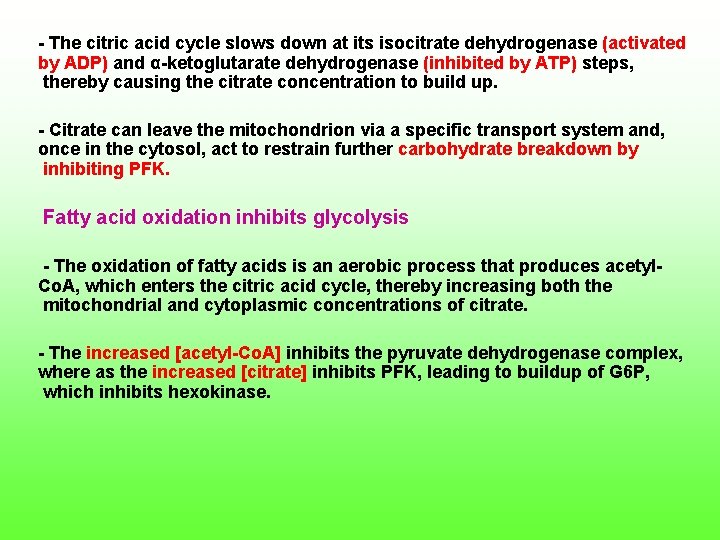
The citric acid cycle slows down at its isocitrate dehydrogenase (activated by ADP) and α ketoglutarate dehydrogenase (inhibited by ATP) steps, thereby causing the citrate concentration to build up. Citrate can leave the mitochondrion via a specific transport system and, once in the cytosol, act to restrain further carbohydrate breakdown by inhibiting PFK. Fatty acid oxidation inhibits glycolysis The oxidation of fatty acids is an aerobic process that produces acetyl Co. A, which enters the citric acid cycle, thereby increasing both the mitochondrial and cytoplasmic concentrations of citrate. The increased [acetyl Co. A] inhibits the pyruvate dehydrogenase complex, where as the increased [citrate] inhibits PFK, leading to buildup of G 6 P, which inhibits hexokinase.

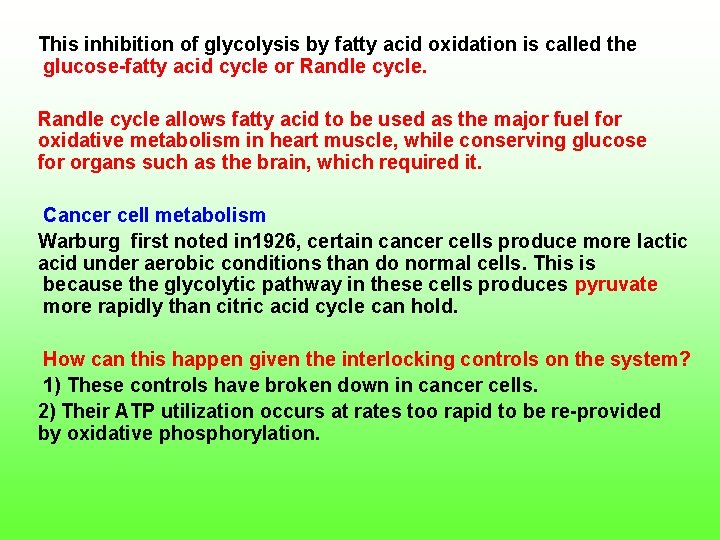
This inhibition of glycolysis by fatty acid oxidation is called the glucose fatty acid cycle or Randle cycle allows fatty acid to be used as the major fuel for oxidative metabolism in heart muscle, while conserving glucose for organs such as the brain, which required it. Cancer cell metabolism Warburg first noted in 1926, certain cancer cells produce more lactic acid under aerobic conditions than do normal cells. This is because the glycolytic pathway in these cells produces pyruvate more rapidly than citric acid cycle can hold. How can this happen given the interlocking controls on the system? 1) These controls have broken down in cancer cells. 2) Their ATP utilization occurs at rates too rapid to be re provided by oxidative phosphorylation.

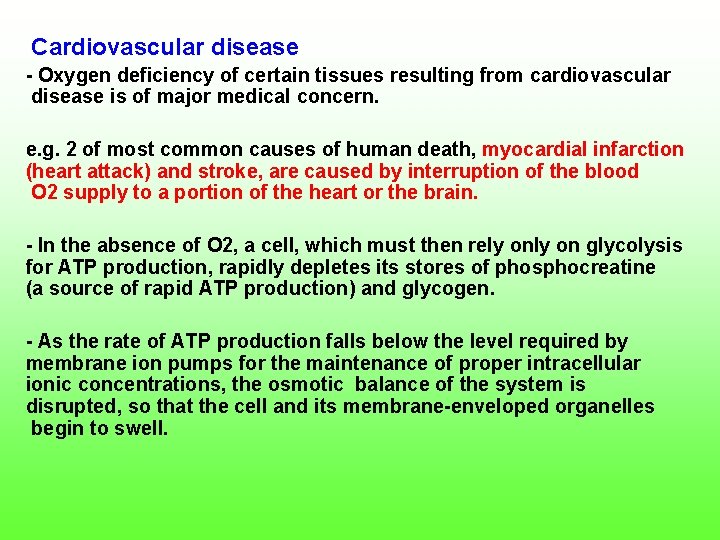
Cardiovascular disease Oxygen deficiency of certain tissues resulting from cardiovascular disease is of major medical concern. e. g. 2 of most common causes of human death, myocardial infarction (heart attack) and stroke, are caused by interruption of the blood O 2 supply to a portion of the heart or the brain. In the absence of O 2, a cell, which must then rely on glycolysis for ATP production, rapidly depletes its stores of phosphocreatine (a source of rapid ATP production) and glycogen. As the rate of ATP production falls below the level required by membrane ion pumps for the maintenance of proper intracellular ionic concentrations, the osmotic balance of the system is disrupted, so that the cell and its membrane enveloped organelles begin to swell.

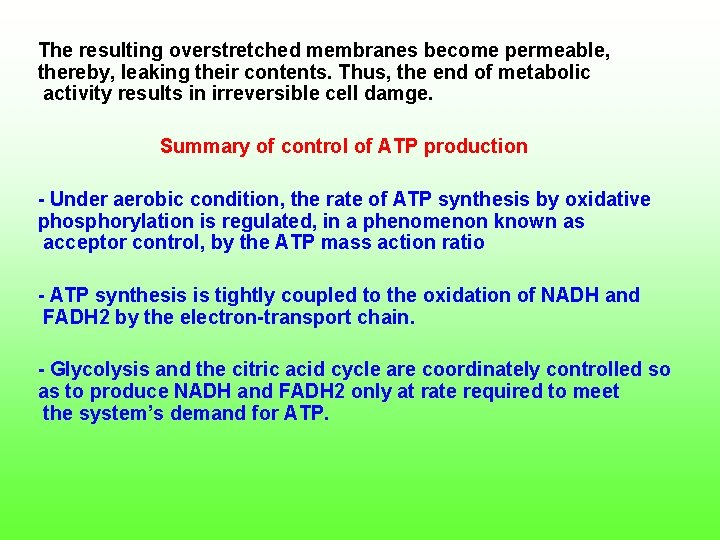
The resulting overstretched membranes become permeable, thereby, leaking their contents. Thus, the end of metabolic activity results in irreversible cell damge. Summary of control of ATP production Under aerobic condition, the rate of ATP synthesis by oxidative phosphorylation is regulated, in a phenomenon known as acceptor control, by the ATP mass action ratio ATP synthesis is tightly coupled to the oxidation of NADH and FADH 2 by the electron transport chain. Glycolysis and the citric acid cycle are coordinately controlled so as to produce NADH and FADH 2 only at rate required to meet the system’s demand for ATP.





