Chromatography Chromatography means of separating and tentatively identifying


- Slides: 9

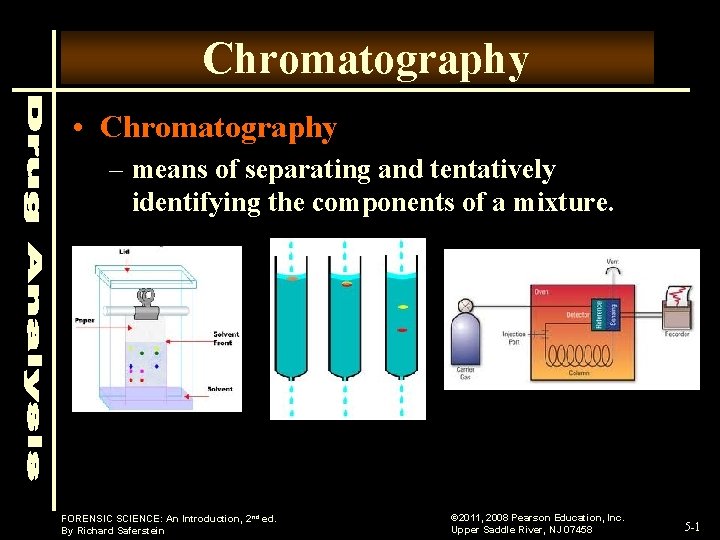
Chromatography • Chromatography – means of separating and tentatively identifying the components of a mixture. FORENSIC SCIENCE: An Introduction, 2 nd ed. By Richard Saferstein © 2011, 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458 5 -1

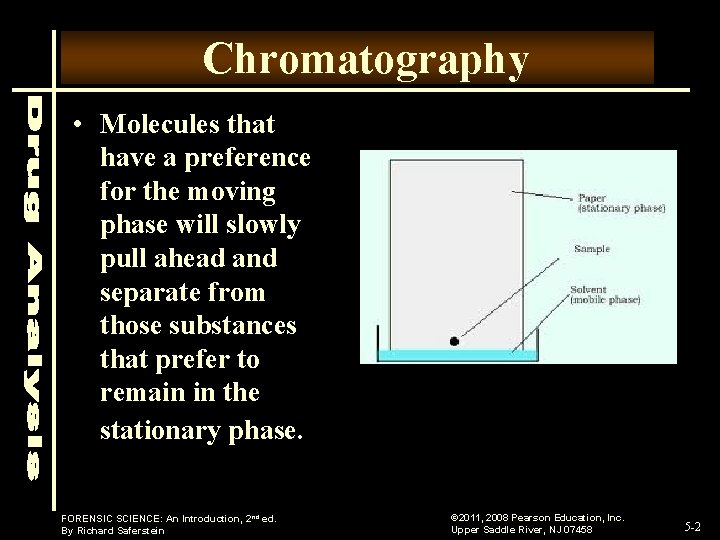
Chromatography • Molecules that have a preference for the moving phase will slowly pull ahead and separate from those substances that prefer to remain in the stationary phase. FORENSIC SCIENCE: An Introduction, 2 nd ed. By Richard Saferstein © 2011, 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458 5 -2

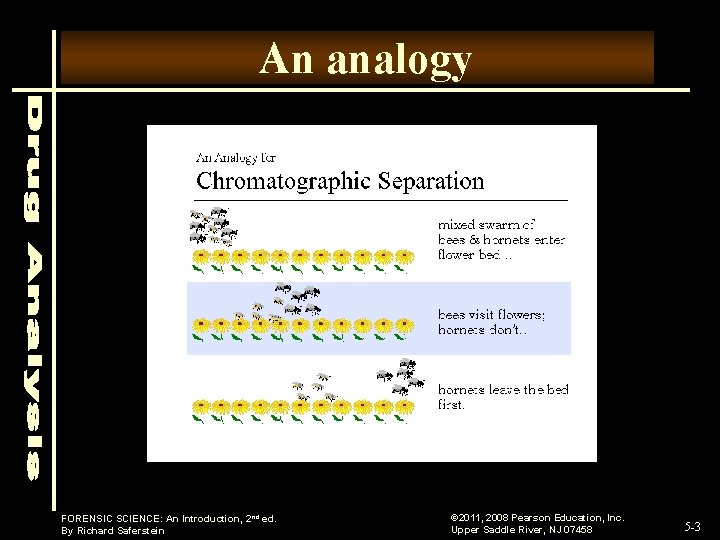
An analogy FORENSIC SCIENCE: An Introduction, 2 nd ed. By Richard Saferstein © 2011, 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458 5 -3

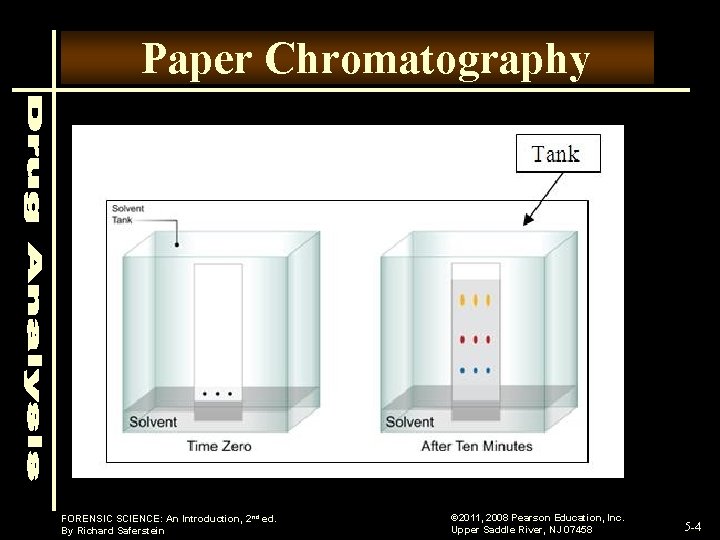
Paper Chromatography FORENSIC SCIENCE: An Introduction, 2 nd ed. By Richard Saferstein © 2011, 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458 5 -4

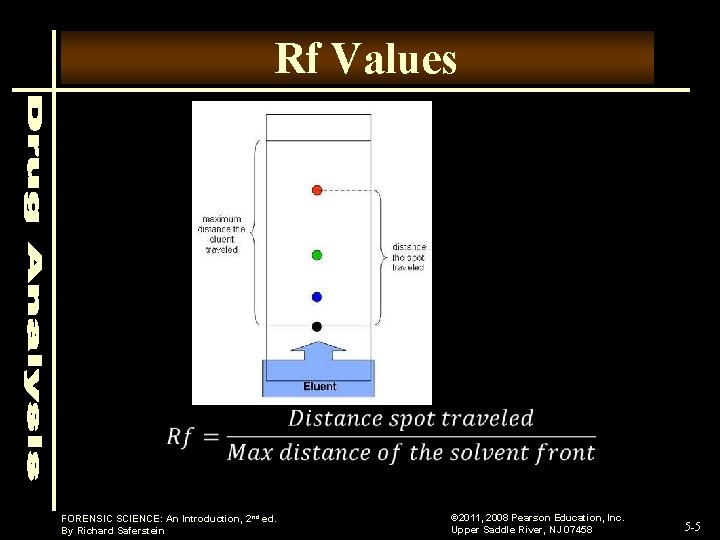
Rf Values FORENSIC SCIENCE: An Introduction, 2 nd ed. By Richard Saferstein © 2011, 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458 5 -5

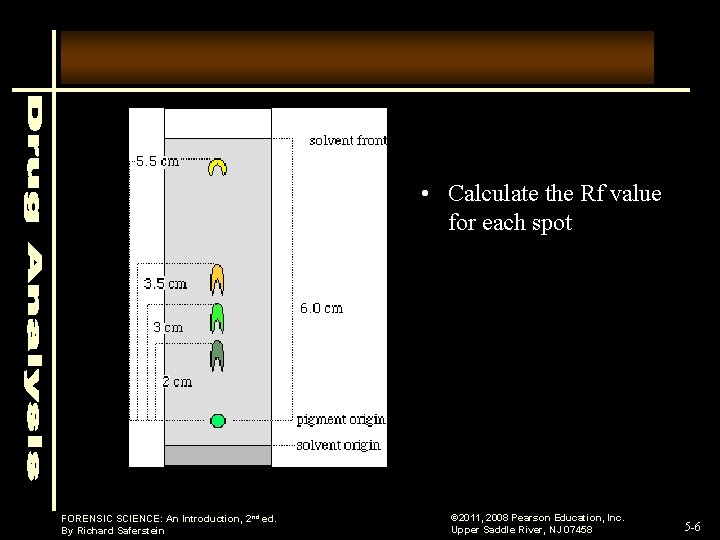
• Calculate the Rf value for each spot FORENSIC SCIENCE: An Introduction, 2 nd ed. By Richard Saferstein © 2011, 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458 5 -6

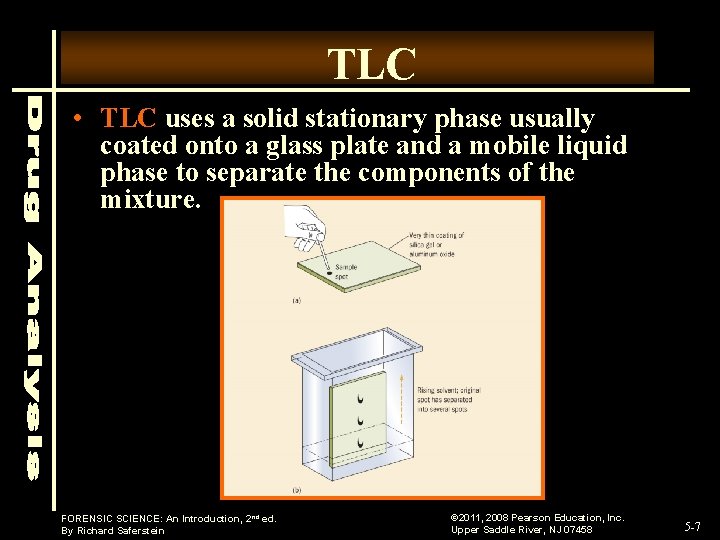
TLC • TLC uses a solid stationary phase usually coated onto a glass plate and a mobile liquid phase to separate the components of the mixture. FORENSIC SCIENCE: An Introduction, 2 nd ed. By Richard Saferstein © 2011, 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458 5 -7

TLC • The liquid will slowly rise up the plate by capillary action – the sample to become distributed between the stationary phase and the moving liquid phase. • Most compounds are colorless – materials must be visualized by placing the plates under ultraviolet light or spraying the plate with a chemical reagent. • Rf values calculated. FORENSIC SCIENCE: An Introduction, 2 nd ed. By Richard Saferstein © 2011, 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458 5 -8

Gas Chromatography • Research gas chromatography. Create a brochure individually that answers the following questions. – What is gas chromatography (GC)? – How does GC work? (Find a visual to help illustrate this) Describe using a few sentences. – What does a gas chromatograph set up look like? What are the parts? Find or draw a visual and label! – What is the written record or data from a GC look like? What is it called? Find an example and print it. – Why/how is GC used in Forensic Science? FORENSIC SCIENCE: An Introduction, 2 nd ed. By Richard Saferstein © 2011, 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458 5 -9
 State your path
State your path What is the method
What is the method Identifying and non identifying adjective clauses
Identifying and non identifying adjective clauses Whats an adjective clause
Whats an adjective clause Identifying and non identifying adjective clauses
Identifying and non identifying adjective clauses Chromatography separating mixtures
Chromatography separating mixtures Chromatography means
Chromatography means Column chromatography images
Column chromatography images Quadrilateral pentagon hexagon octagon
Quadrilateral pentagon hexagon octagon Meta means change and morph means heat
Meta means change and morph means heat