CHROMATOGRAPHY Chromatography basically involves the separation of mixtures

CHROMATOGRAPHY Chromatography basically involves the separation of mixtures due to differences in the distribution coefficient (equilibrium distribution) of sample components between 2 different phases. One of these phases is a mobile phase and the other is a stationary phase.

Distribution Coefficient (Equilibrium Distribution ) Definition: Concentration of component A in stationary phase Concentration of component A in mobile phase Different affinity of these 2 components to stationary phase causes the separation.

Kinds of Chromatography 1. Liquid Column Chromatography 2. Gas Liquid Chromatography 3. Thin-layer Chromatography

LIQUID COLUMN CHROMATOGRAPHY A sample mixture is passed through a column packed with solid particles which may or may not be coated with another liquid. With the proper solvents, packing conditions, some components in the sample will travel the column more slowly than others resulting in the desired separation.
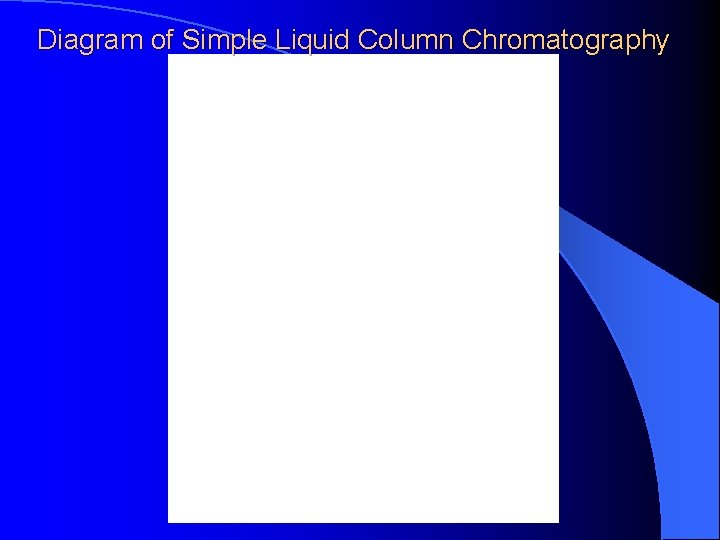
Diagram of Simple Liquid Column Chromatography

FOUR BASIC LIQUID CHROMATOGRAPHY The 4 basic liquid chromatography modes are named according to the mechanism involved: 1. Liquid/Solid Chromatography (adsorption chromatography) A. Normal Phase LSC B. Reverse Phase LSC 2. Liquid/Liquid Chromatography (partition chromatography) A. Normal Phase LLC B. Reverse Phase LLC 3. Ion Exchange Chromatography 4. Gel Permeation Chromatography (exclusion chromatography)
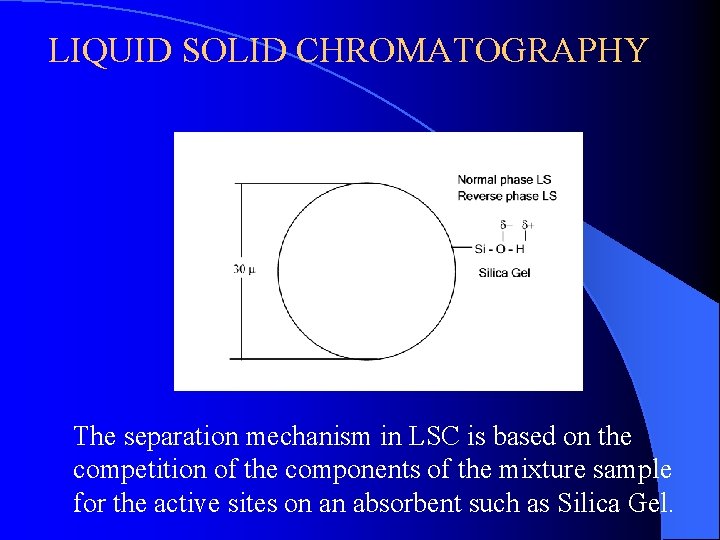
LIQUID SOLID CHROMATOGRAPHY The separation mechanism in LSC is based on the competition of the components of the mixture sample for the active sites on an absorbent such as Silica Gel.
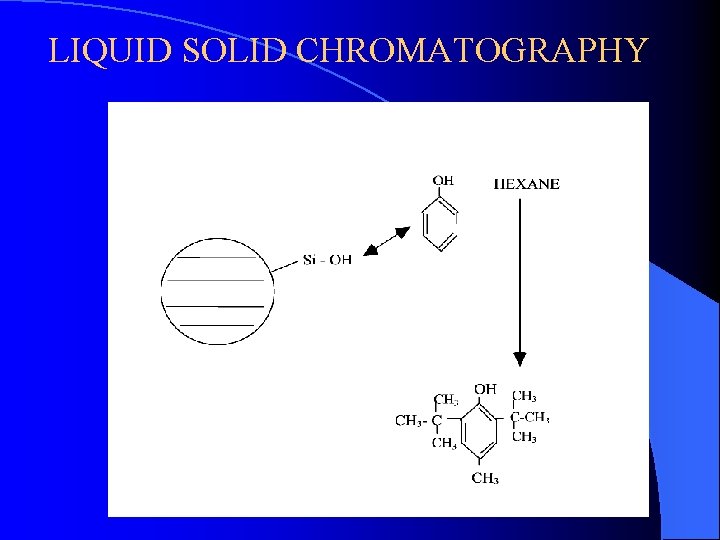
LIQUID SOLID CHROMATOGRAPHY
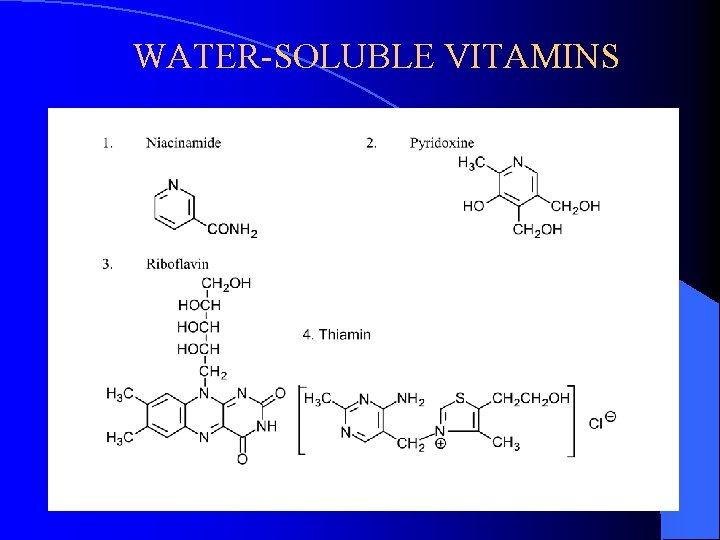
WATER-SOLUBLE VITAMINS

WATER-SOLUBLE VITAMINS
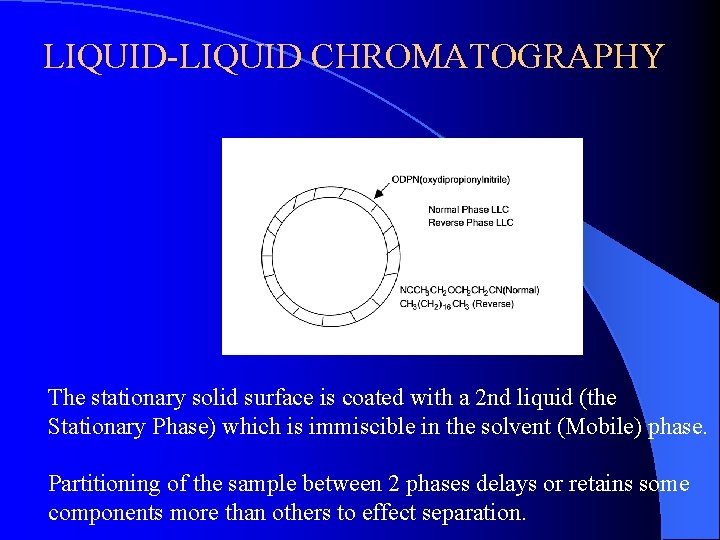
LIQUID-LIQUID CHROMATOGRAPHY The stationary solid surface is coated with a 2 nd liquid (the Stationary Phase) which is immiscible in the solvent (Mobile) phase. Partitioning of the sample between 2 phases delays or retains some components more than others to effect separation.
Types of Chromatography
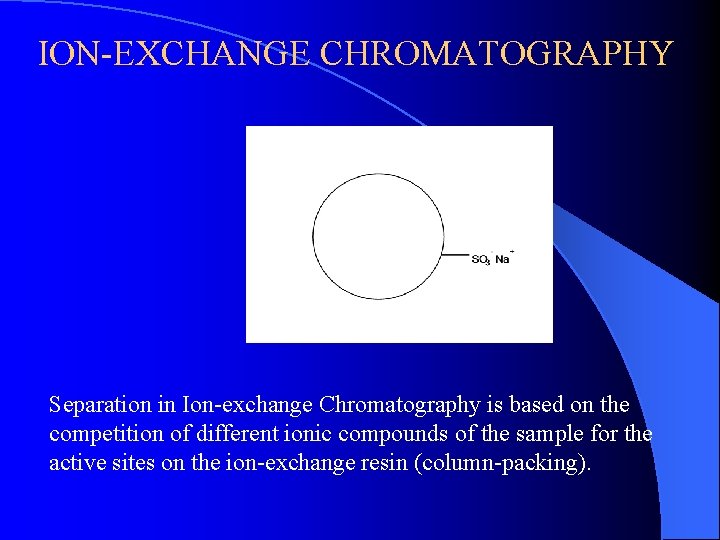
ION-EXCHANGE CHROMATOGRAPHY Separation in Ion-exchange Chromatography is based on the competition of different ionic compounds of the sample for the active sites on the ion-exchange resin (column-packing).
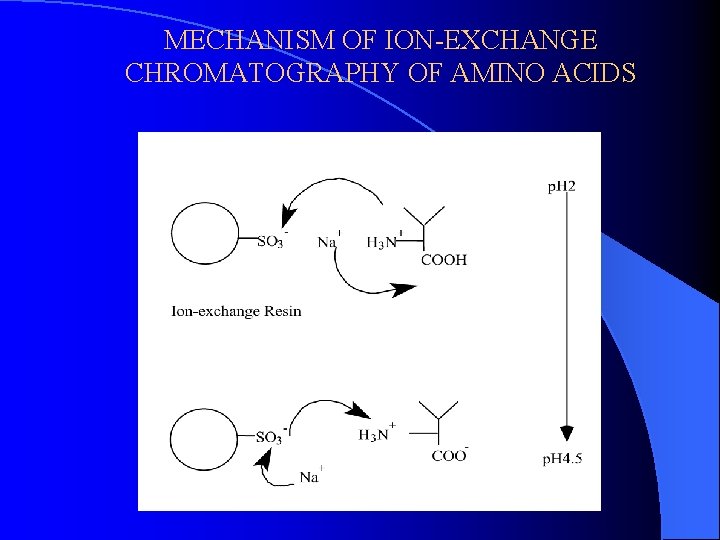
MECHANISM OF ION-EXCHANGE CHROMATOGRAPHY OF AMINO ACIDS
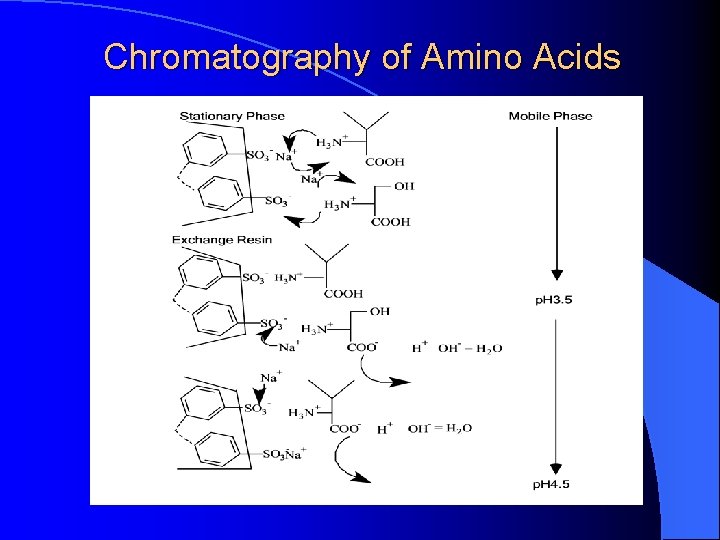
Chromatography of Amino Acids
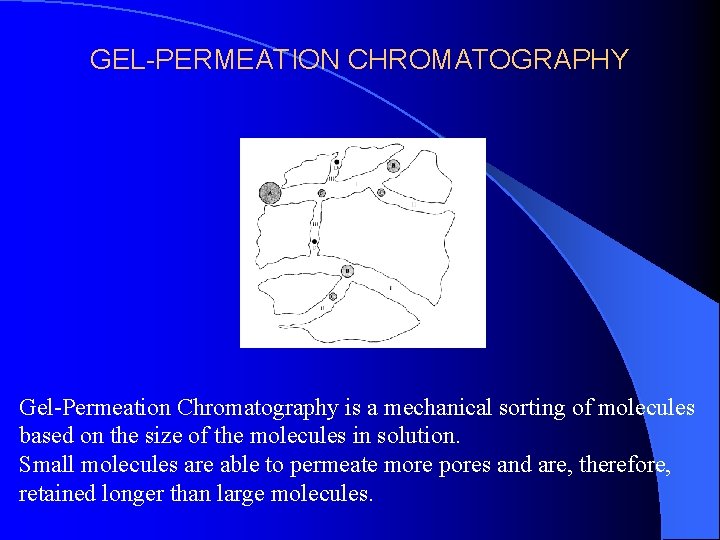
GEL-PERMEATION CHROMATOGRAPHY Gel-Permeation Chromatography is a mechanical sorting of molecules based on the size of the molecules in solution. Small molecules are able to permeate more pores and are, therefore, retained longer than large molecules.

SOLVENTS Polar Solvents Water > Methanol > Acetonitrile > Ethanol > Oxydipropionitrile Non-polar Solvents N-Decane > N-Hexane > N-Pentane > Cyclohexane
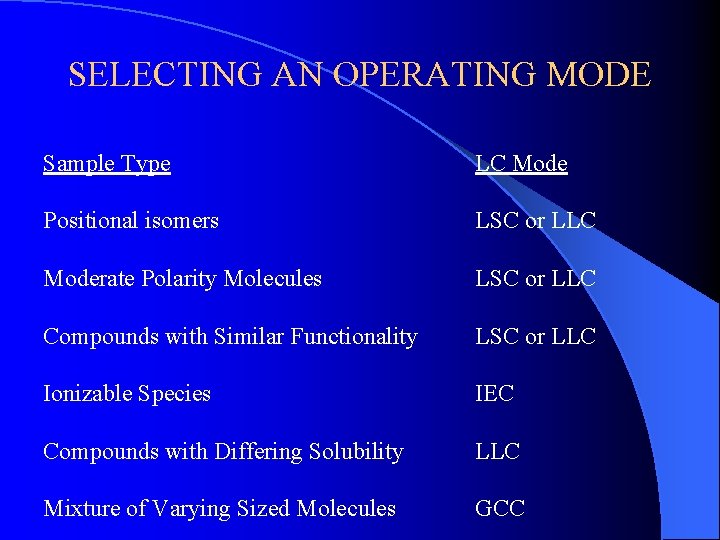
SELECTING AN OPERATING MODE Sample Type LC Mode Positional isomers LSC or LLC Moderate Polarity Molecules LSC or LLC Compounds with Similar Functionality LSC or LLC Ionizable Species IEC Compounds with Differing Solubility LLC Mixture of Varying Sized Molecules GCC
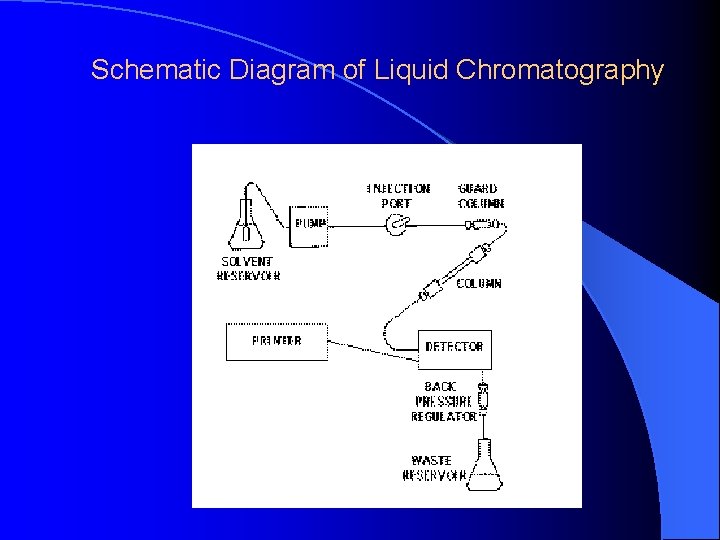
Schematic Diagram of Liquid Chromatography
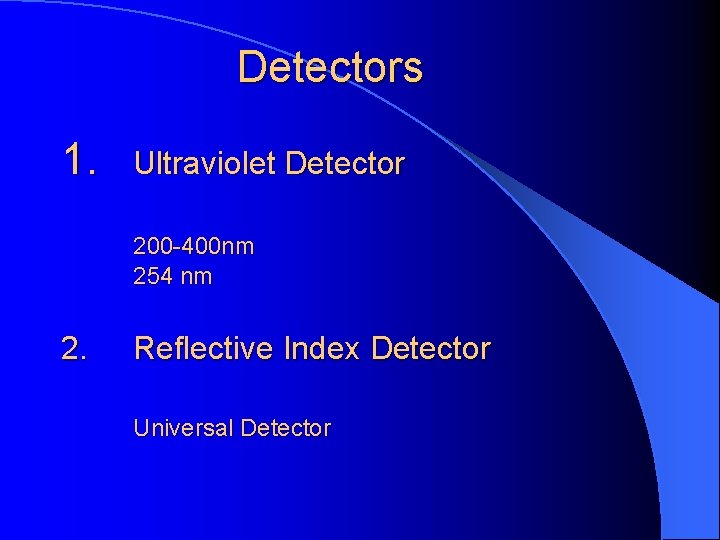
Detectors 1. Ultraviolet Detector 200 -400 nm 254 nm 2. Reflective Index Detector Universal Detector

High Performance Liquid Chromatography

High Performance Liquid Chromatography
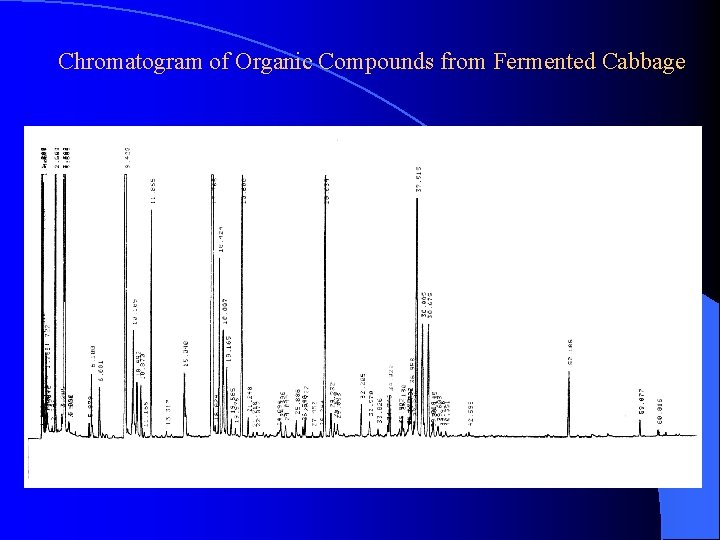
Chromatogram of Organic Compounds from Fermented Cabbage
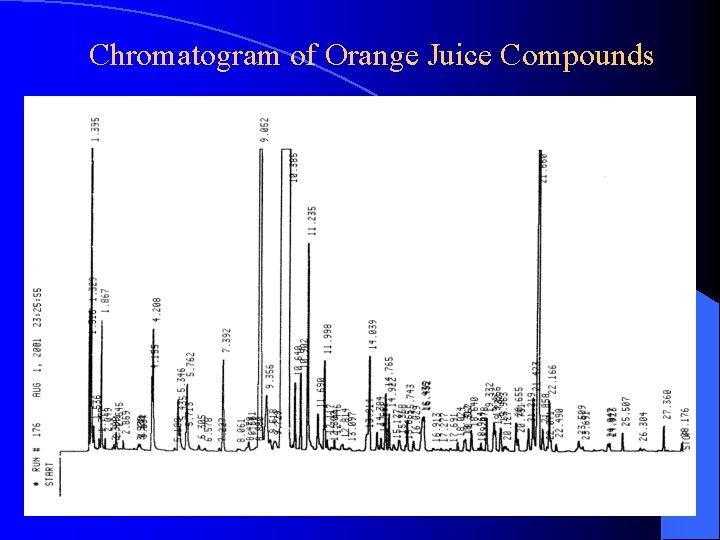
Chromatogram of Orange Juice Compounds

Retention Time required for the sample to travel from the injection port through the column to the detector.
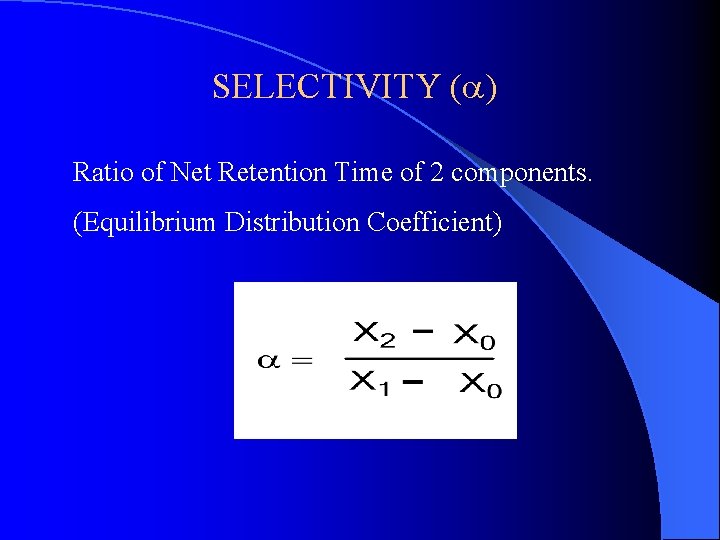
SELECTIVITY (a) Ratio of Net Retention Time of 2 components. (Equilibrium Distribution Coefficient)

Selectivity – Selectivity
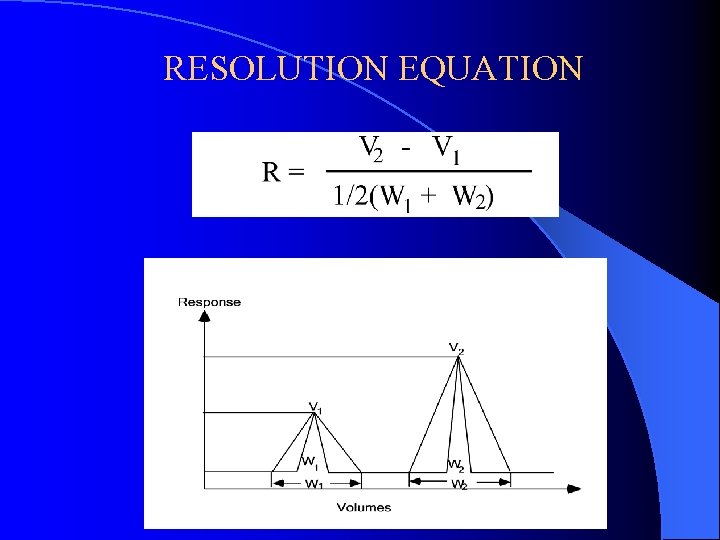
RESOLUTION EQUATION
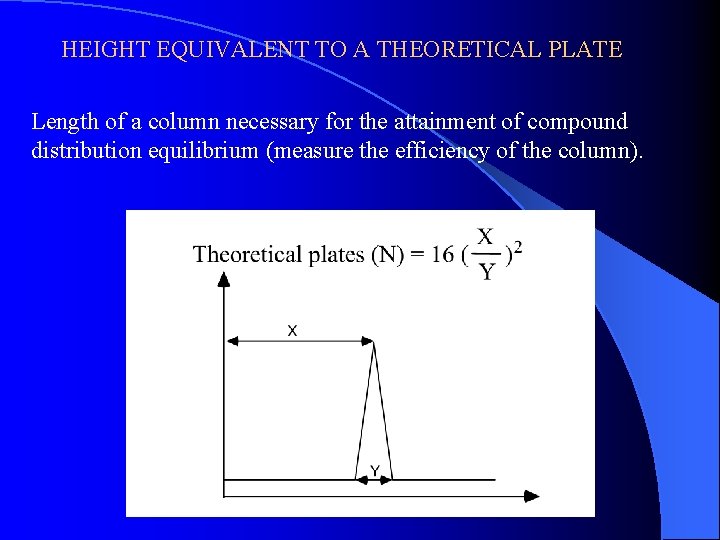
HEIGHT EQUIVALENT TO A THEORETICAL PLATE Length of a column necessary for the attainment of compound distribution equilibrium (measure the efficiency of the column).
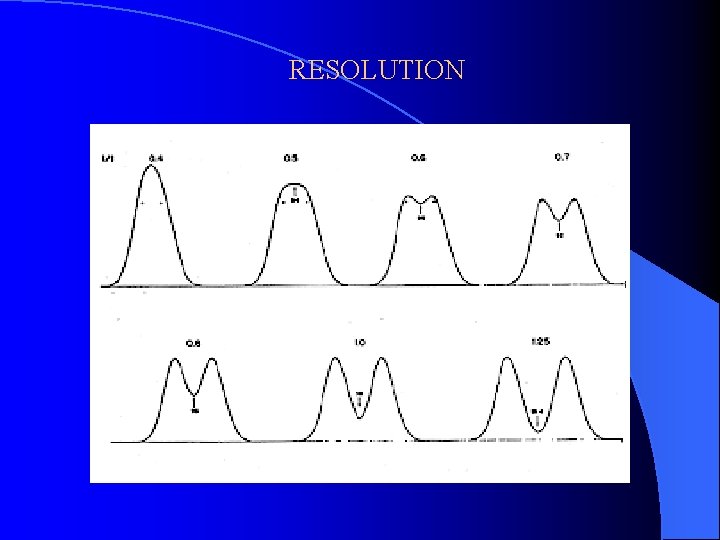
RESOLUTION

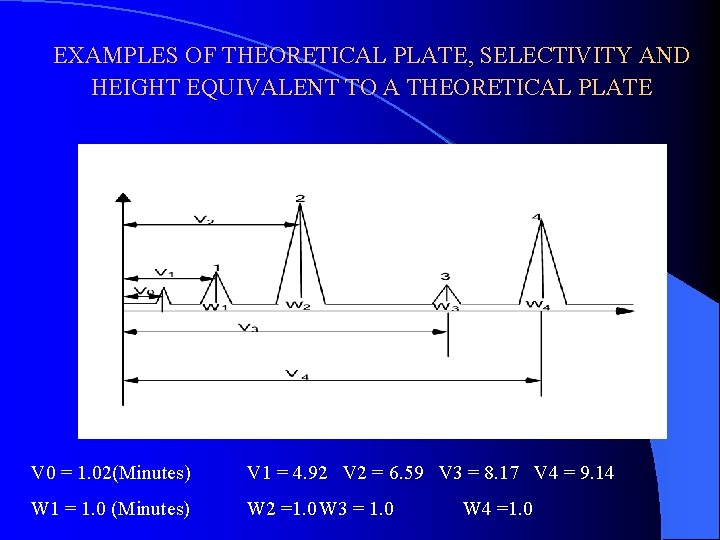
EXAMPLES OF THEORETICAL PLATE, SELECTIVITY AND HEIGHT EQUIVALENT TO A THEORETICAL PLATE V 0 = 1. 02(Minutes) V 1 = 4. 92 V 2 = 6. 59 V 3 = 8. 17 V 4 = 9. 14 W 1 = 1. 0 (Minutes) W 2 =1. 0 W 3 = 1. 0 W 4 =1. 0

GENERAL FACTORS INCREASING RESOLUTION 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Increase column length Decrease column diameter Decrease flow-rate Pack column uniformly Use uniform stationary phase (packing material) Decrease sample size Select proper stationary phase Select proper mobile phase Use proper pressure Use gradient elution
- Slides: 34