Chpt 2 The Atom History of the Atom













- Slides: 31

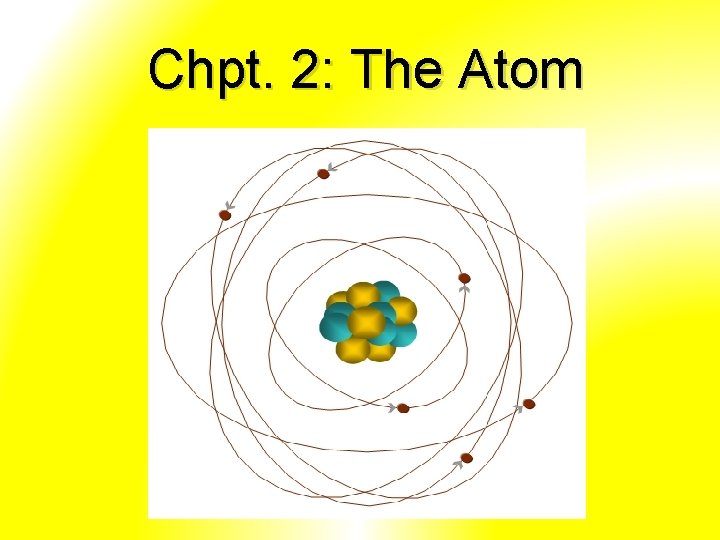
Chpt. 2: The Atom

History of the Atom 1. Greek Philosophers (400 BC): - first proposed that matter was composed of minute particles - believed that the tiny particles of which all matter was composed were so small that nothing smaller was possible ‘Atomos’ Greek word for indivisible - ATOM

2. John Dalton (1808): Dalton’s Atomic Theory - All matter is made up of very small particles called atoms - All atoms are indivisible. They cannot be broken down into simpler particles - Atoms cannot be created or destroyed

What is inside the atom? ? ?

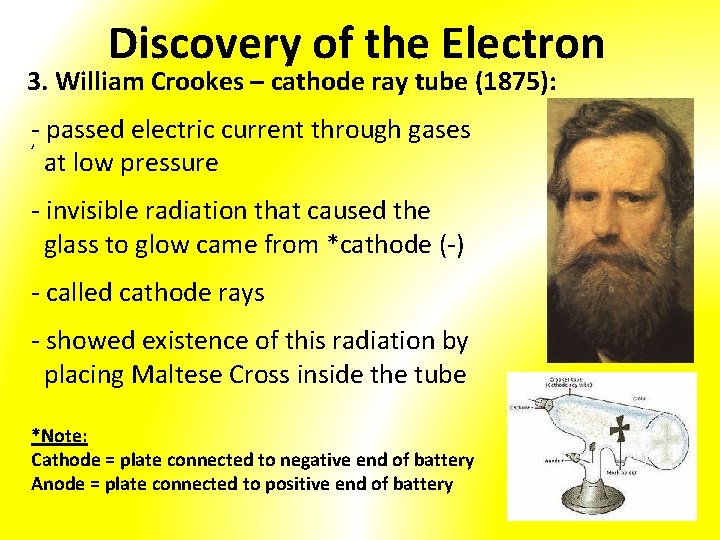
Discovery of the Electron 3. William Crookes – cathode ray tube (1875): -, passed electric current through gases at low pressure - invisible radiation that caused the glass to glow came from *cathode (-) - called cathode rays - showed existence of this radiation by placing Maltese Cross inside the tube *Note: Cathode = plate connected to negative end of battery Anode = plate connected to positive end of battery


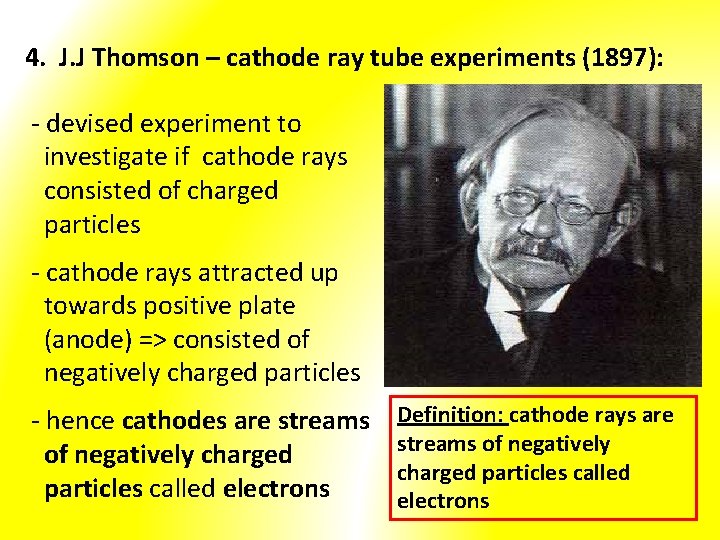
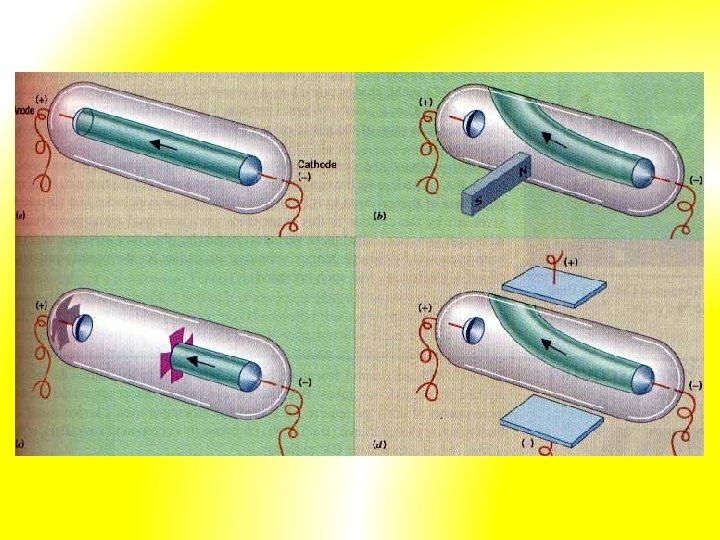
4. J. J Thomson – cathode ray tube experiments (1897): - devised experiment to investigate if cathode rays consisted of charged particles - cathode rays attracted up towards positive plate (anode) => consisted of negatively charged particles - hence cathodes are streams of negatively charged particles called electrons Definition: cathode rays are streams of negatively charged particles called electrons

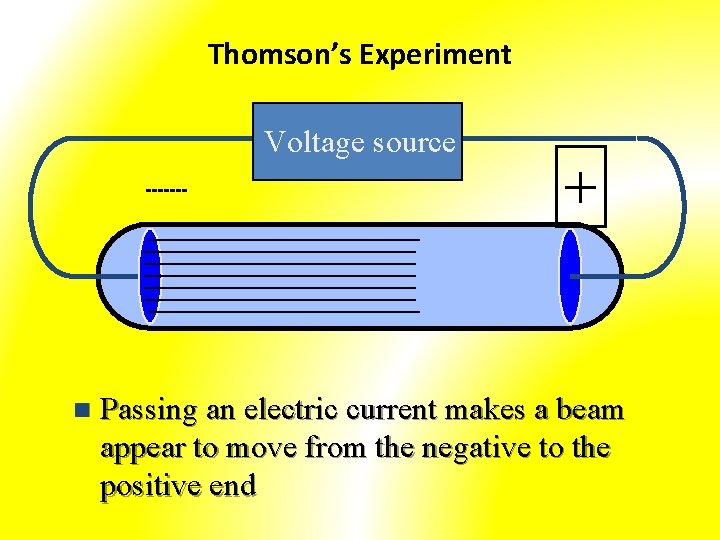
Thomson’s Experiment Voltage source ------- n + Passing an electric current makes a beam appear to move from the negative to the positive end

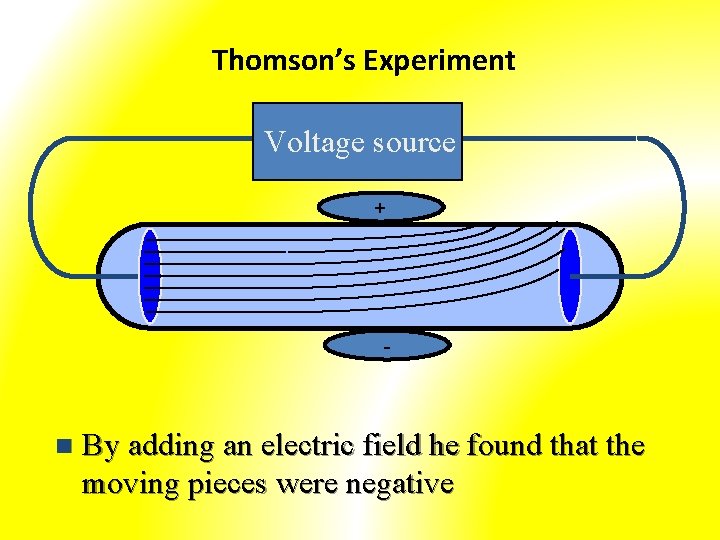
Thomson’s Experiment Voltage source + - n By adding an electric field he found that the moving pieces were negative

Further experiment: - he found electrons were also deflected in magnetic field - found ratio of charge to mass of the electron (e/m): (electrical charge of electron) (mass of electron) = 1. 76 x 108 coulombs = 1 gram of electrons *Note: In 1891 George Stoney proposed that the smallest amount of electric charge be called an electron.


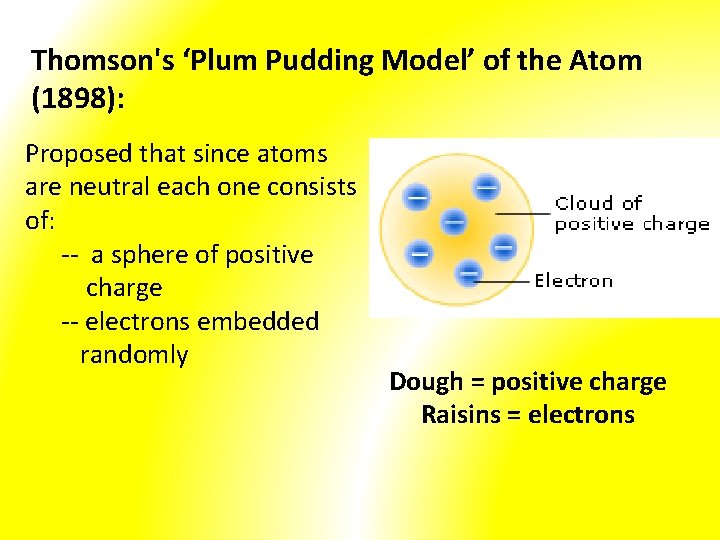
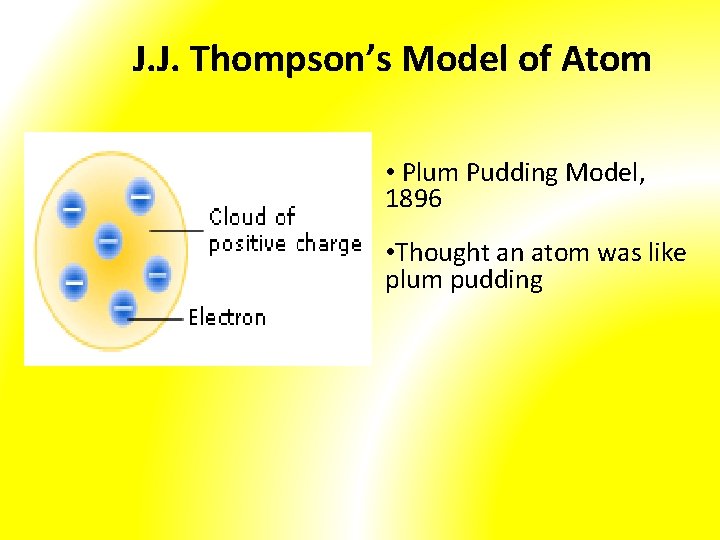
Thomson's ‘Plum Pudding Model’ of the Atom (1898): Proposed that since atoms are neutral each one consists of: -- a sphere of positive charge -- electrons embedded randomly Dough = positive charge Raisins = electrons

5. Robert Millikan (1909): - Experiment to measure size of charge on electron – Oil Drop Experiment - Charge of one electron = 1. 6 x 10 -19 coulomb THUS…. Mass of e- = 9. 11 x 10 -31 g

Discovery of radiation led to the use of alpha particles in experiments Alpha particles are positively charged particles produced by certain radioactive substances

6. Ernest Rutherford (1909): Rutherford discovered the nucleus and the proton

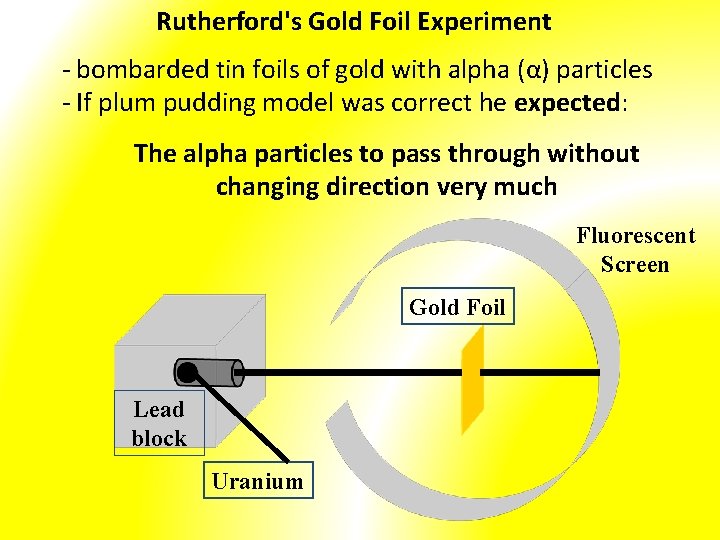
Rutherford's Gold Foil Experiment - bombarded tin foils of gold with alpha (α) particles - If plum pudding model was correct he expected: The alpha particles to pass through without changing direction very much Fluorescent Screen Gold Foil Lead block Uranium

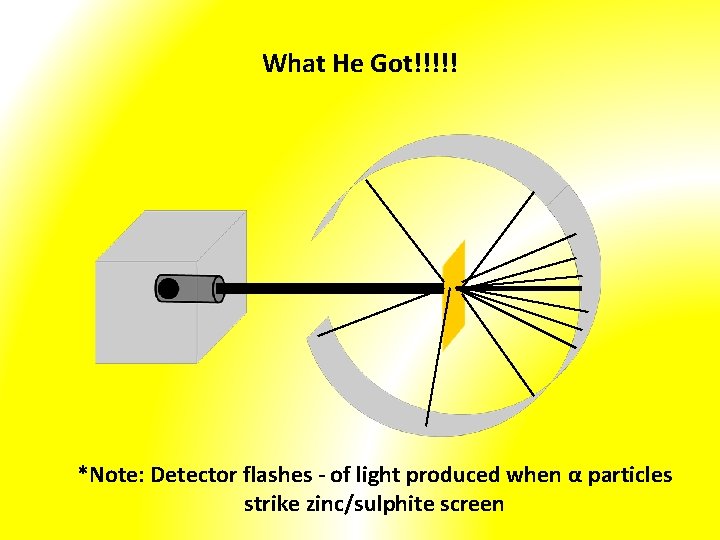
What He Got!!!!! *Note: Detector flashes - of light produced when α particles strike zinc/sulphite screen

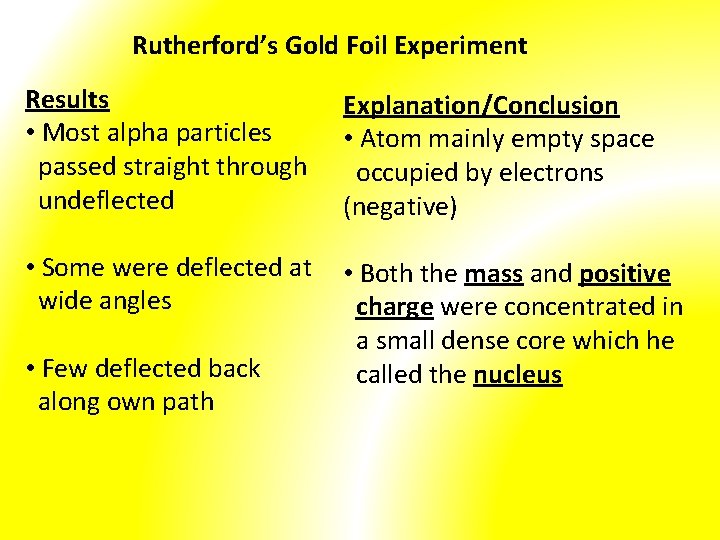
Rutherford’s Gold Foil Experiment Results • Most alpha particles passed straight through undeflected Explanation/Conclusion • Atom mainly empty space occupied by electrons (negative) • Some were deflected at wide angles • Both the mass and positive charge were concentrated in a small dense core which he called the nucleus • Few deflected back along own path

Rutherford – discovery of protons (1924): • Light atoms (oxygen, nitrogen) were bombarded with alpha particles - small POSITIVE charged particles were given off • This did not occur with heavier metals e. g. gold • Explanation – alpha particles were breaking up the nuclei of the lighter atoms to release positively charged particles • referred to these small positive particles as protons

7. James Chadwick (1932): • Search for a neutral particle to cement the nucleus • Bombarded beryllium with alpha particles • Produced neutral particles – neutron

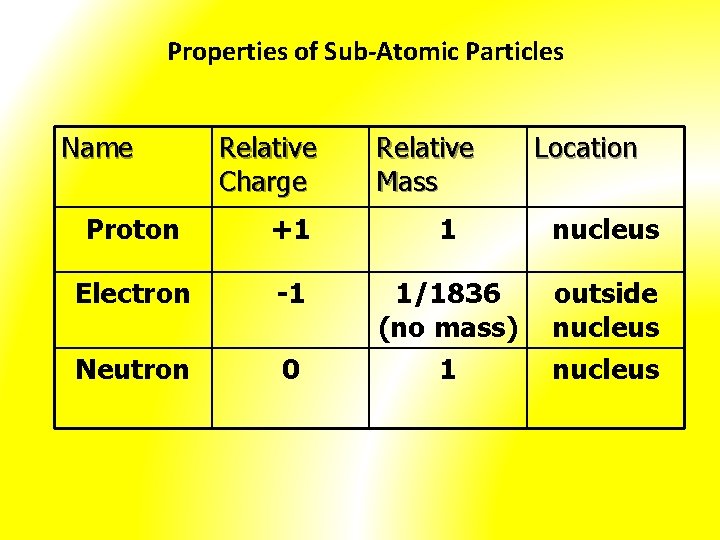
Properties of Sub-Atomic Particles Name Relative Charge Relative Mass Location Proton +1 1 nucleus Electron -1 1/1836 (no mass) outside nucleus Neutron 0 1 nucleus

Dalton Model of the Atom • Small, indivisible spheres

J. J. Thompson’s Model of Atom • Plum Pudding Model, 1896 • Thought an atom was like plum pudding

Rutherford’s Model of the Atom • Rutherford Model, 1911 • Thought atom was mostly empty space: - Nucleus - Electrons (negatively charged) revolving around nucleus

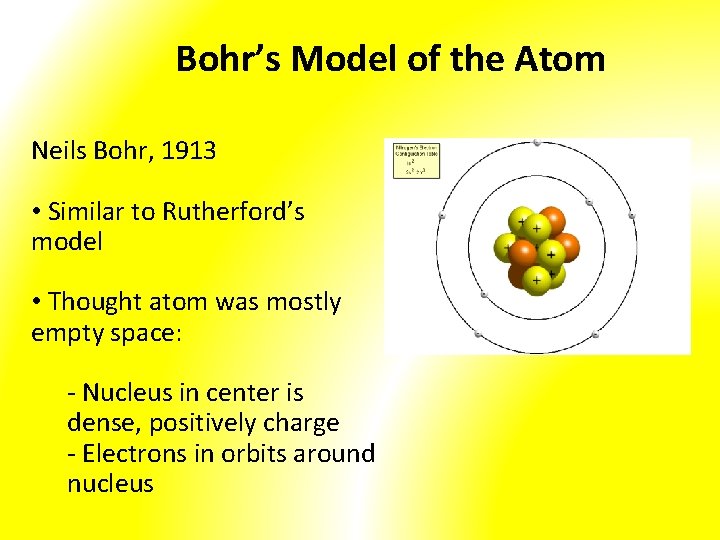
Bohr’s Model of the Atom Neils Bohr, 1913 • Similar to Rutherford’s model • Thought atom was mostly empty space: - Nucleus in center is dense, positively charge - Electrons in orbits around nucleus

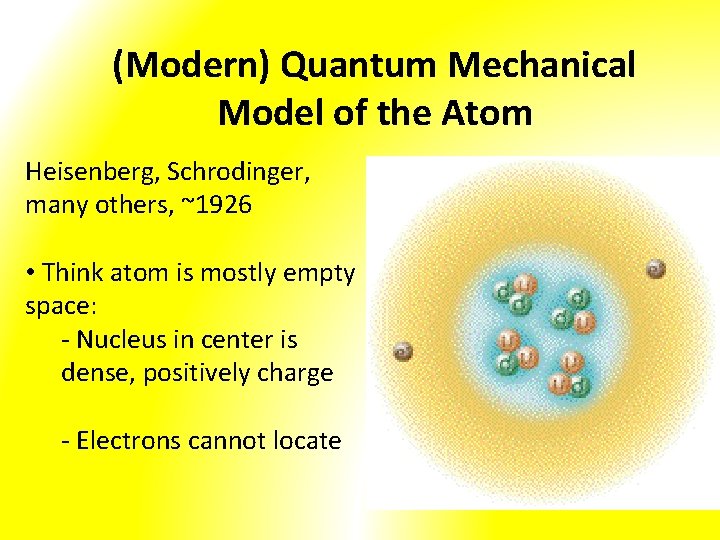
(Modern) Quantum Mechanical Model of the Atom Heisenberg, Schrodinger, many others, ~1926 • Think atom is mostly empty space: - Nucleus in center is dense, positively charge - Electrons cannot locate

Evidence for the existence of small particles!!! Why is it possible to smell the perfume that someone is wearing from several metres away?

Diffusion • The process by which molecules of a substance spread through a solid, liquid or gas. • Some examples which can be demonstrated in the lab: - Gas Jar full of air

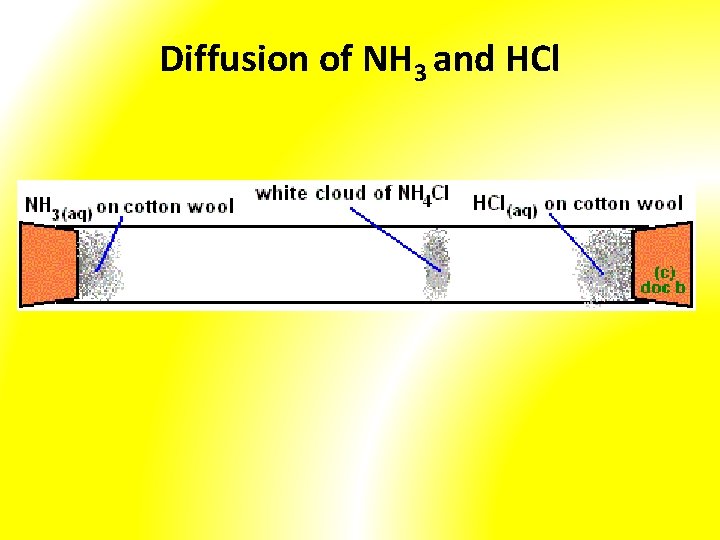
Demonstration • Diffusion of ink in water • Diffusion of NH 3 and HCl • Diffusion of smoke in air

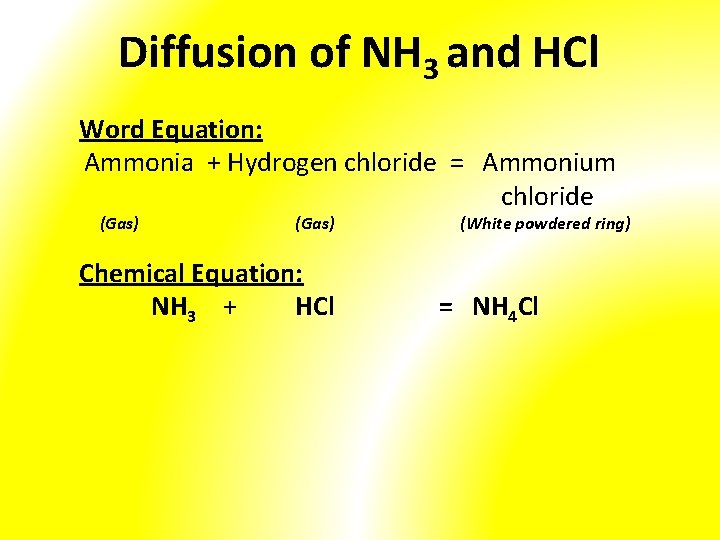
Diffusion of NH 3 and HCl

Diffusion of NH 3 and HCl Word Equation: Ammonia + Hydrogen chloride = Ammonium chloride (Gas) Chemical Equation: NH 3 + HCl (White powdered ring) = NH 4 Cl
 Questionnaire design process chpt 11
Questionnaire design process chpt 11 Questionnaire design process chpt 11
Questionnaire design process chpt 11 Chpt er
Chpt er Chpt er
Chpt er The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Teori atom democritus
Teori atom democritus History of the atom webquest
History of the atom webquest Atomic model history
Atomic model history Democritus atomic model
Democritus atomic model History of the atom john dalton
History of the atom john dalton Teori atom democritus
Teori atom democritus Atom models history
Atom models history Bohr model vs quantum model venn diagram
Bohr model vs quantum model venn diagram History also history physical
History also history physical Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worms-breton
Tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra