Chp 3 Matter I Classification of Matter A










- Slides: 19

Chp. #3 - Matter I. Classification of Matter

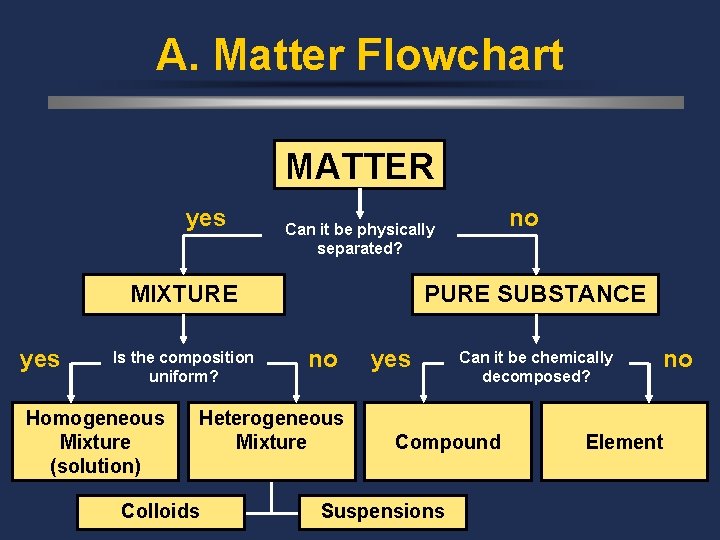
A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions no Element

B. Pure Substances ö Element w composed of identical atoms w can’t be separated by physical or chemical means w EX: copper wire, aluminum foil

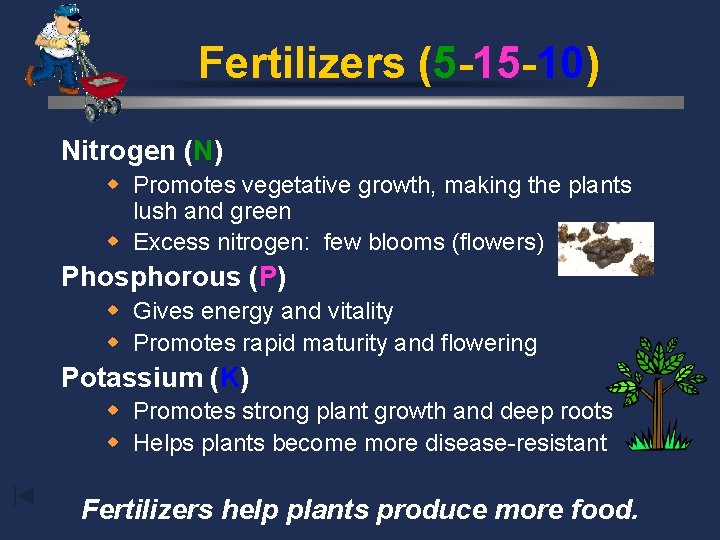
Fertilizers (5 -15 -10) Nitrogen (N) w Promotes vegetative growth, making the plants lush and green w Excess nitrogen: few blooms (flowers) Phosphorous (P) w Gives energy and vitality w Promotes rapid maturity and flowering Potassium (K) w Promotes strong plant growth and deep roots w Helps plants become more disease-resistant Fertilizers help plants produce more food.

B. Pure Substances ö Compound w composed of 2 or more elements in a fixed ratio w properties differ from those of individual elements w EX: table salt (Na. Cl) Water ( H 2 O)

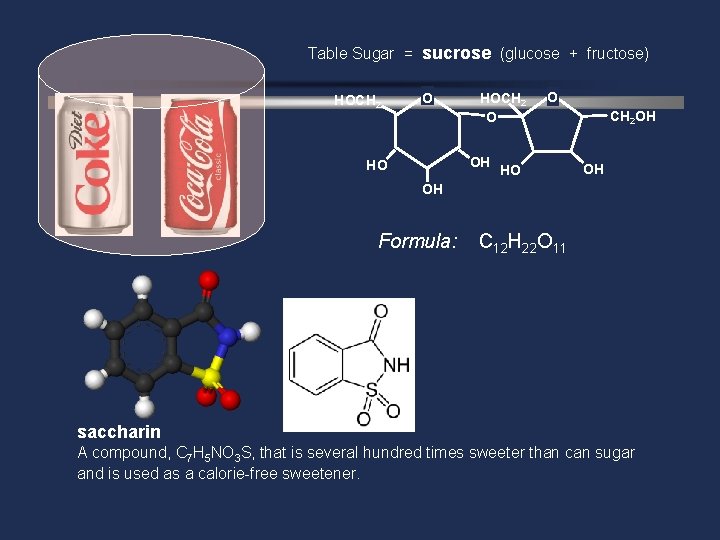
Table Sugar = sucrose (glucose + fructose) HOCH 2 O OH HO O HO CH 2 OH OH OH Formula: C 12 H 22 O 11 saccharin A compound, C 7 H 5 NO 3 S, that is several hundred times sweeter than can sugar and is used as a calorie-free sweetener.

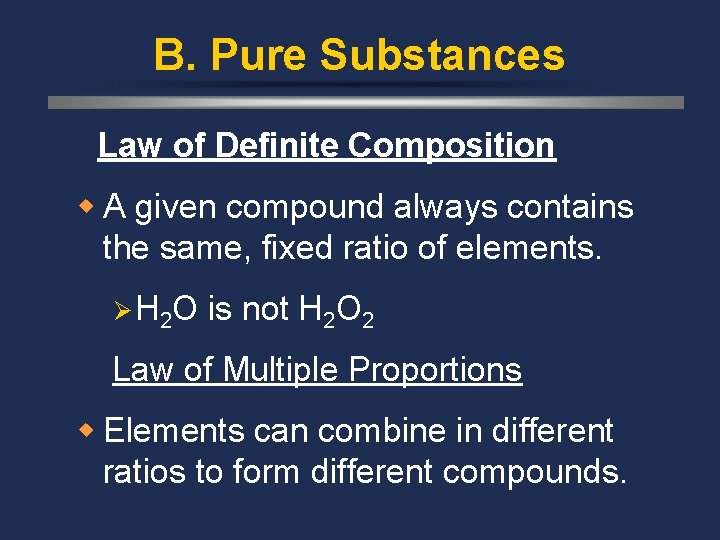
B. Pure Substances Law of Definite Composition w A given compound always contains the same, fixed ratio of elements. Ø H 2 O is not H 2 O 2 Law of Multiple Proportions w Elements can combine in different ratios to form different compounds.

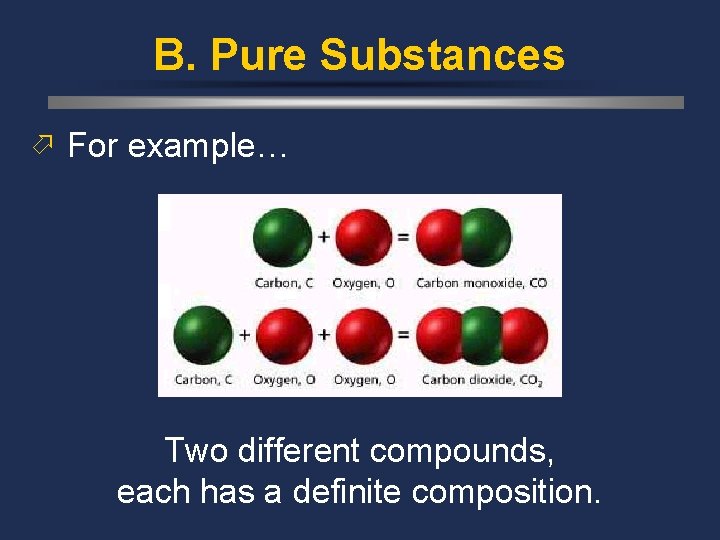
B. Pure Substances ö For example… Two different compounds, each has a definite composition.

C. Mixtures ö Variable combination of 2 or more pure substances. Heterogeneous Homogeneous

C. Mixtures ö Solution w homogeneous w very small particles w no Tyndall effect w particles don’t settle w EX: rubbing alcohol sugar water Tyndall Effect

C. Mixtures ö Colloid w heterogeneous w medium-sized particles w Tyndall effect w particles don’t settle w EX: milk

C. Mixtures ö Suspension w heterogeneous w large particles w Tyndall effect w particles settle w EX: fresh-squeezed lemonade

D. Examples ö Examples: w graphite Element (carbon) w pepper hetero. mixture w sugar (sucrose) Compound(C 12 H 22 O 11) w paint Suspension (hetero) w soda Solution (homog. )

ONE OF THESE Properties of Matter CHEMICAL properties tell how a substance reacts with other substances. PHYSICAL properties can be observed without chemically changing the substance. EXTENSIVE properties depend on the amount of substance present (volume, mass etc. ) INTENSIVE properties do NOT depend on the amount of substance but the identity(density). ONE OF THESE AND

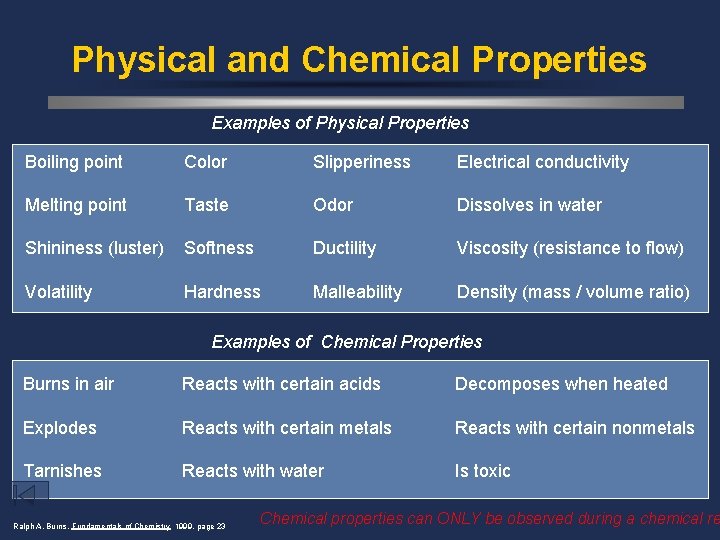
Physical and Chemical Properties Examples of Physical Properties Boiling point Color Slipperiness Electrical conductivity Melting point Taste Odor Dissolves in water Shininess (luster) Softness Ductility Viscosity (resistance to flow) Volatility Hardness Malleability Density (mass / volume ratio) Examples of Chemical Properties Burns in air Reacts with certain acids Decomposes when heated Explodes Reacts with certain metals Reacts with certain nonmetals Tarnishes Reacts with water Is toxic Ralph A. Burns, Fundamentals of Chemistry 1999, page 23 Chemical properties can ONLY be observed during a chemical re

Bell Ringer (for next class) ö Classify using the matter flowchart: w water Compound w muddy water Suspension (hetero) w fog Colloid (heterog. ) w saltwater Solution (homog) w Hydrogen gas element

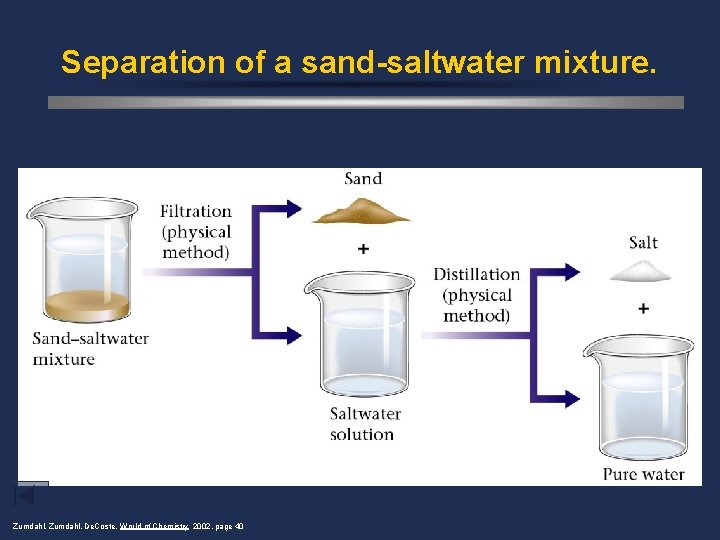
Separation of a sand-saltwater mixture. Zumdahl, De. Coste, World of Chemistry 2002, page 40

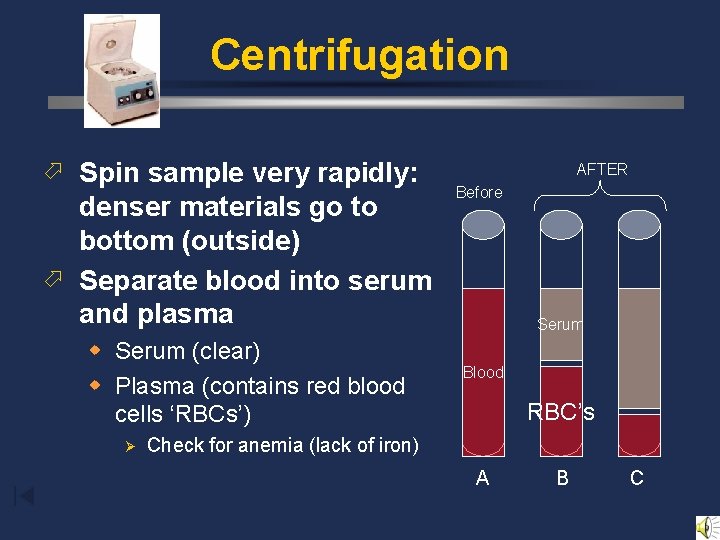
Centrifugation ö Spin sample very rapidly: denser materials go to bottom (outside) ö Separate blood into serum and plasma w Serum (clear) w Plasma (contains red blood cells ‘RBCs’) Ø AFTER Before Serum Blood RBC’s Check for anemia (lack of iron) A B C

Define and Explain these methods of Separation - pp. 476 -478 etc. ö Filtration – ö Magnetism – ö Crystallization – ö Chromatography – ö Distillation – ö Electrolysisö Decantingö Centrifugeö Evaporation-
 Chp bit inspection
Chp bit inspection Yenifer chp vr
Yenifer chp vr Chp
Chp Acute variables of training
Acute variables of training What are the 5 major discourses in matthew
What are the 5 major discourses in matthew Introduction to hand tools module 3 answers
Introduction to hand tools module 3 answers Chp mission statement
Chp mission statement Double ended queue
Double ended queue Chp 14
Chp 14 Decp
Decp Tipo de selección natural
Tipo de selección natural Chp christiansburg va
Chp christiansburg va Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Gray matter in the brain
Gray matter in the brain Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Arbor vitae
Arbor vitae Gray matter and white matter
Gray matter and white matter Matter
Matter