Choosing and using LEL PID and NDIR Sensors








- Slides: 50

Choosing and using LEL, PID and NDIR Sensors and Applications Bob Henderson President Gf. G Instrumentation 1194 Oak Valley Drive Ann Arbor, MI 48108 Toll free: (800) 950 -0329 Direct: (734) 769 -0573 Fax: (734) 769 -1888 E-mail: info@gfg-inc. com Internet: www. gfg-inc. com July 2010 Using LEL, PID and IR Sensors Slide 1

Learning Objectives • Capabilities and limitations of LEL, infrared (NDIR) and photoionization (PID) sensors • Using EC toxic sensors for the new toxic exposure limits for H 2 S and SO 2 July 2010 Using LEL, PID and IR Sensors Slide 2

Traditional EC Toxic and Combustible Pellistor sensors • More types of sensors available every year, both for individual toxic gases as well as sensors designed to detect a range of toxic or combustible gases July 2010 Using LEL, PID and IR Sensors Slide 3

Substance-specific electrochemical sensors • Gas diffusing into sensor reacts at surface of the sensing electrode • Sensing electrode made to catalyze a specific reaction • Use of selective external filters further limits cross sensitivity July 2010 Using LEL, PID and IR Sensors Slide 4

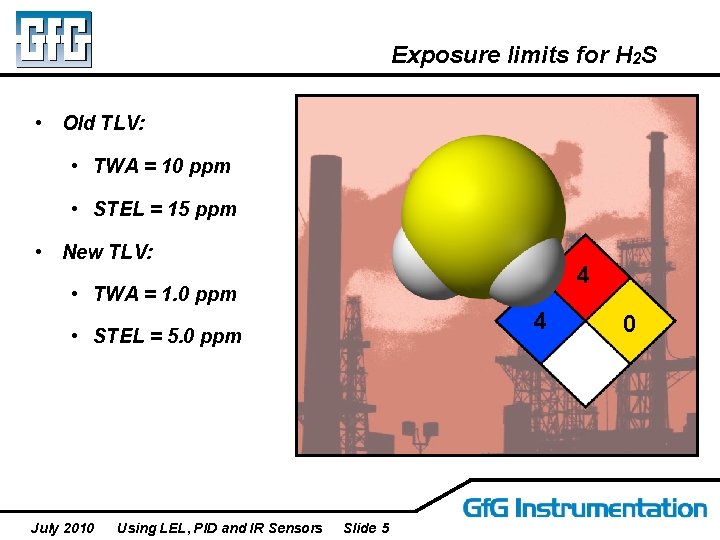
Exposure limits for H 2 S • Old TLV: • TWA = 10 ppm • STEL = 15 ppm • New TLV: 4 • TWA = 1. 0 ppm 4 • STEL = 5. 0 ppm July 2010 Using LEL, PID and IR Sensors Slide 5 0

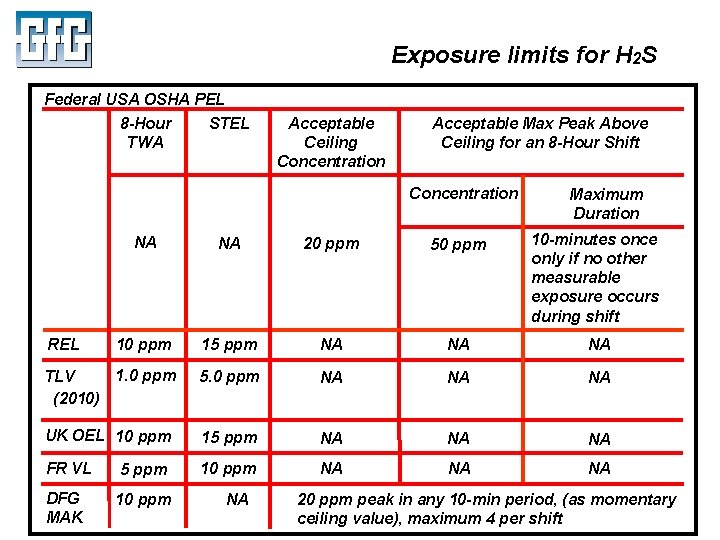
Exposure limits for H 2 S Federal USA OSHA PEL 8 -Hour TWA STEL Acceptable Ceiling Concentration Acceptable Max Peak Above Ceiling for an 8 -Hour Shift Concentration Maximum Duration NA NA 20 ppm 50 ppm 10 -minutes once only if no other measurable exposure occurs during shift 10 ppm 15 ppm NA NA NA 1. 0 ppm TLV (2010) UK OEL 10 ppm 5. 0 ppm NA 15 ppm NA FR VL 5 ppm 10 ppm NA DFG MAK 10 ppm REL July 2010 NA NA 20 ppm peak in any 10 -min period, (as momentary ceiling value), maximum 4 per shift Using LEL, PID and IR Sensors Slide 6

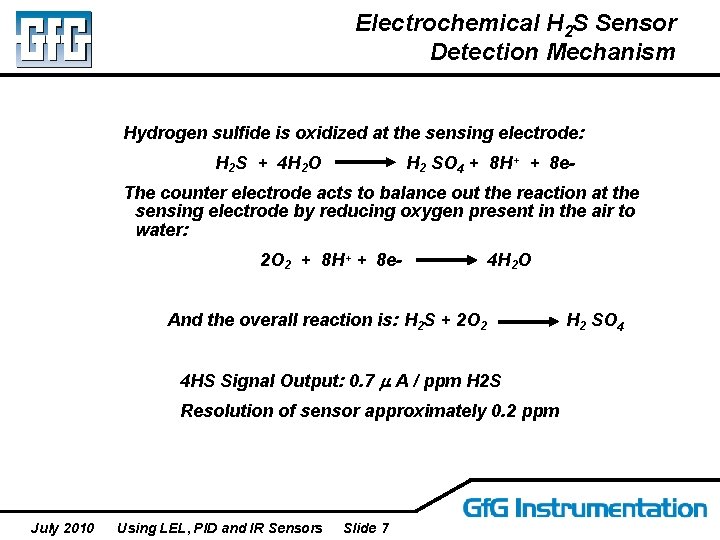
Electrochemical H 2 S Sensor Detection Mechanism Hydrogen sulfide is oxidized at the sensing electrode: H 2 S + 4 H 2 O H 2 SO 4 + 8 H+ + 8 e- The counter electrode acts to balance out the reaction at the sensing electrode by reducing oxygen present in the air to water: 2 O 2 + 8 H+ + 8 e- 4 H 2 O And the overall reaction is: H 2 S + 2 O 2 4 HS Signal Output: 0. 7 A / ppm H 2 S Resolution of sensor approximately 0. 2 ppm July 2010 Using LEL, PID and IR Sensors Slide 7 H 2 SO 4

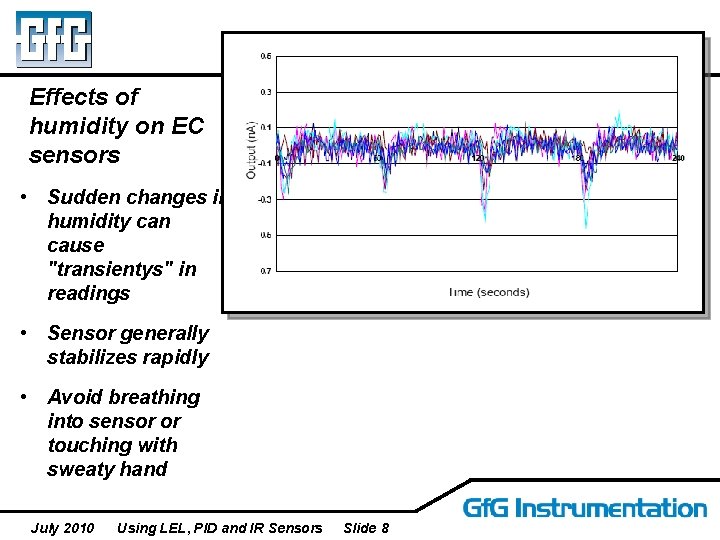
Effects of humidity on EC sensors • Sudden changes in humidity can cause "transientys" in readings • Sensor generally stabilizes rapidly • Avoid breathing into sensor or touching with sweaty hand July 2010 Using LEL, PID and IR Sensors Slide 8

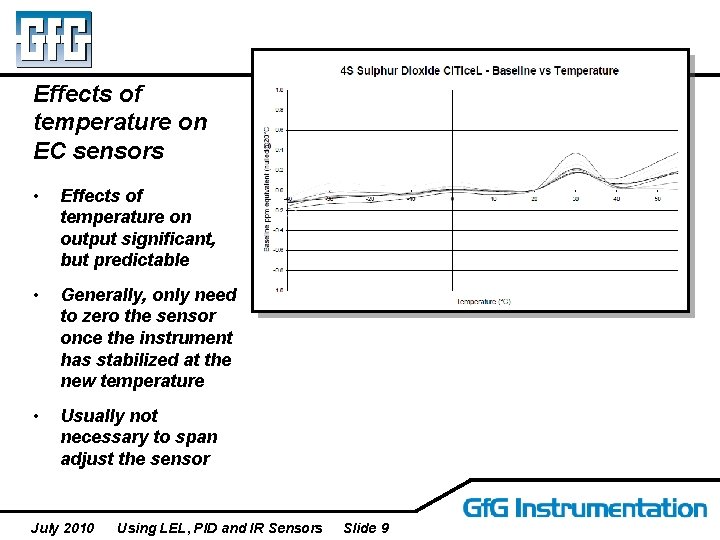
Effects of temperature on EC sensors • Effects of temperature on output significant, but predictable • Generally, only need to zero the sensor once the instrument has stabilized at the new temperature • Usually not necessary to span adjust the sensor July 2010 Using LEL, PID and IR Sensors Slide 9

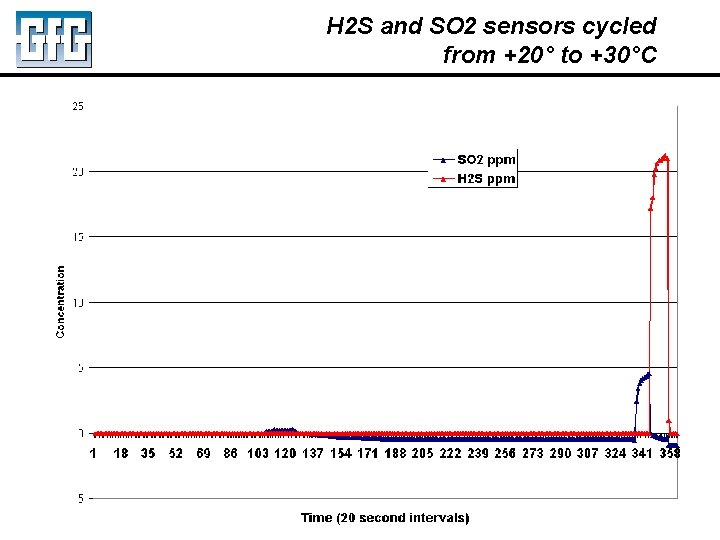
H 2 S and SO 2 sensors cycled from +20° to +30°C July 2010 Using LEL, PID and IR Sensors Slide 10

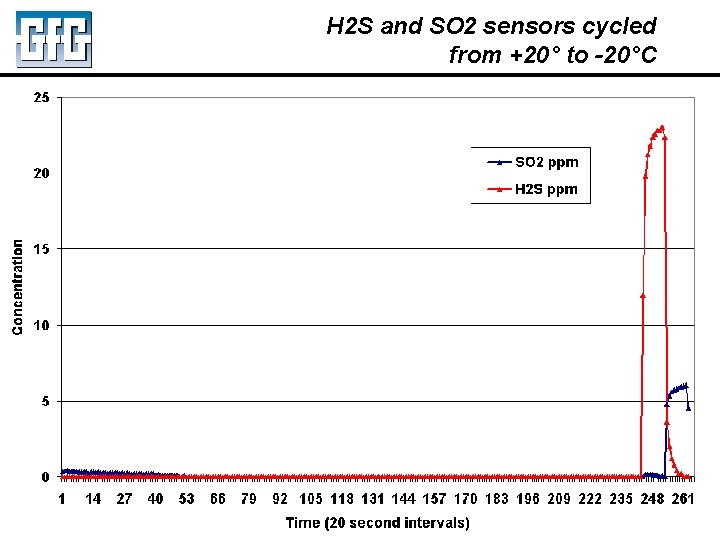
H 2 S and SO 2 sensors cycled from +20° to -20°C July 2010 Using LEL, PID and IR Sensors Slide 11

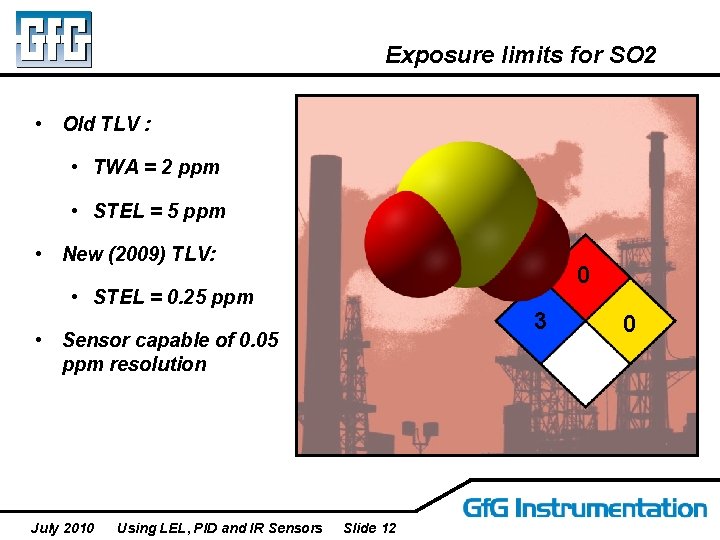
Exposure limits for SO 2 • Old TLV : • TWA = 2 ppm • STEL = 5 ppm • New (2009) TLV: 0 • STEL = 0. 25 ppm 3 • Sensor capable of 0. 05 ppm resolution July 2010 Using LEL, PID and IR Sensors Slide 12 0

Exposure limits for NO 2 • TLV: • 8 hr. TWA = 3 ppm • 15 min. STEL = 5 ppm • US OSHA PEL: 0 • Ceiling = 5 ppm 3 • US NIOSH REL: OX • 15 min. STEL = 1 ppm • Sensor capable of 0. 1 ppm resolution July 2010 Using LEL, PID and IR Sensors 0 Slide 13

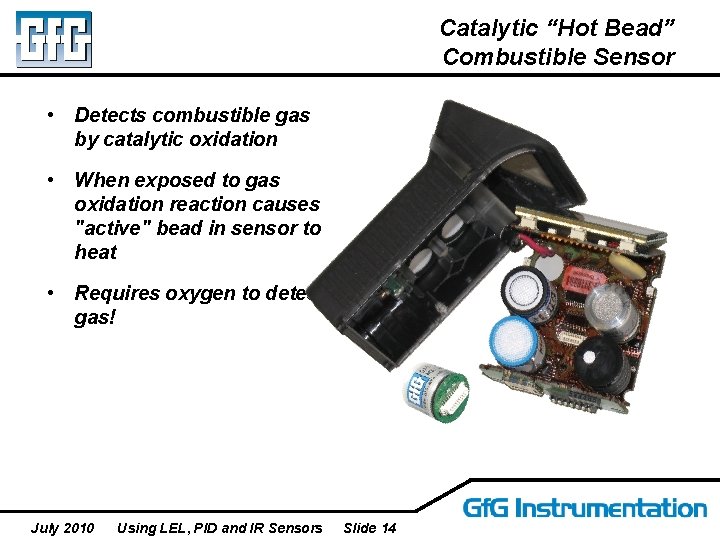
Catalytic “Hot Bead” Combustible Sensor • Detects combustible gas by catalytic oxidation • When exposed to gas oxidation reaction causes "active" bead in sensor to heat • Requires oxygen to detect gas! July 2010 Using LEL, PID and IR Sensors Slide 14

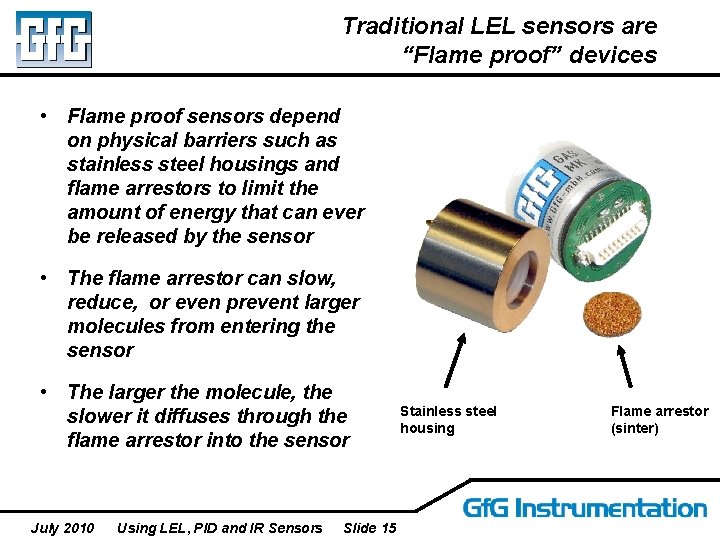
Traditional LEL sensors are “Flame proof” devices • Flame proof sensors depend on physical barriers such as stainless steel housings and flame arrestors to limit the amount of energy that can ever be released by the sensor • The flame arrestor can slow, reduce, or even prevent larger molecules from entering the sensor • The larger the molecule, the slower it diffuses through the flame arrestor into the sensor July 2010 Using LEL, PID and IR Sensors Slide 15 Stainless steel housing Flame arrestor (sinter)

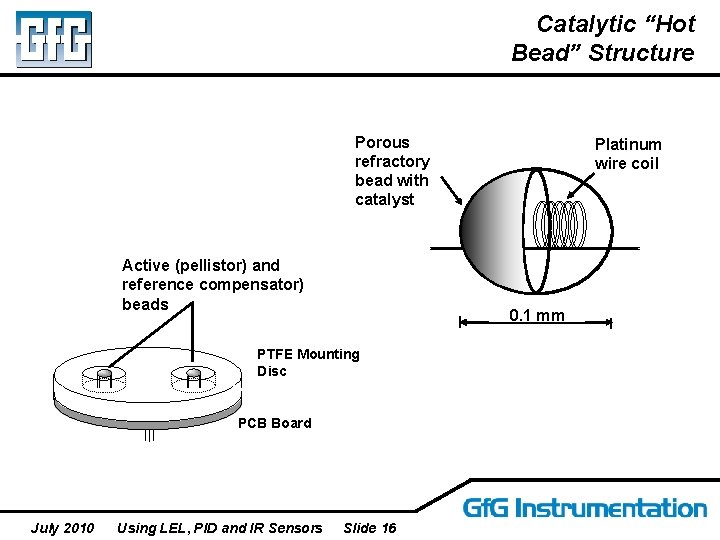
Catalytic “Hot Bead” Structure Porous refractory bead with catalyst Active (pellistor) and reference compensator) beads 0. 1 mm PTFE Mounting Disc PCB Board July 2010 Using LEL, PID and IR Sensors Platinum wire coil Slide 16

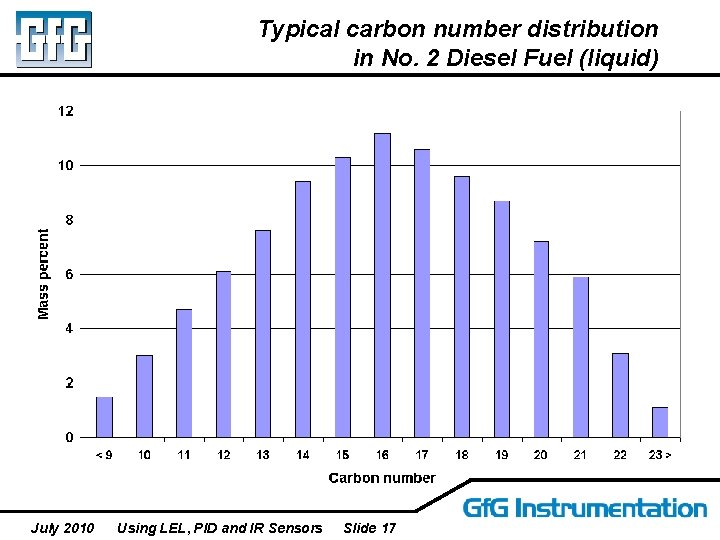
Typical carbon number distribution in No. 2 Diesel Fuel (liquid) July 2010 Using LEL, PID and IR Sensors Slide 17

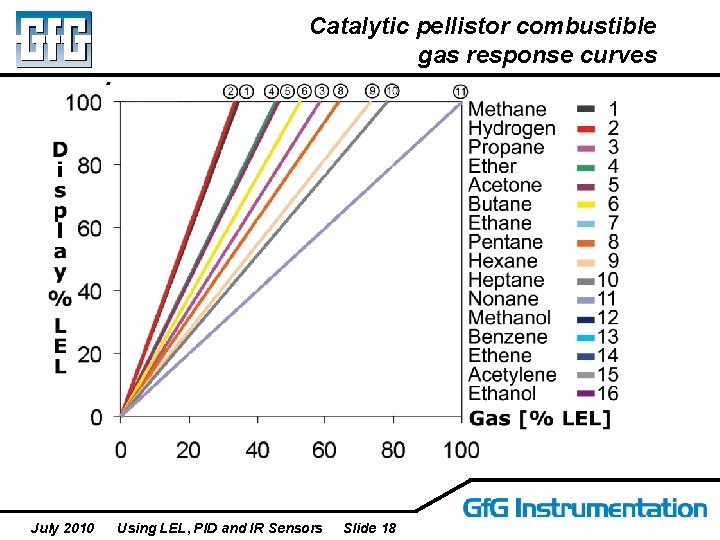
Catalytic pellistor combustible gas response curves July 2010 Using LEL, PID and IR Sensors Slide 18

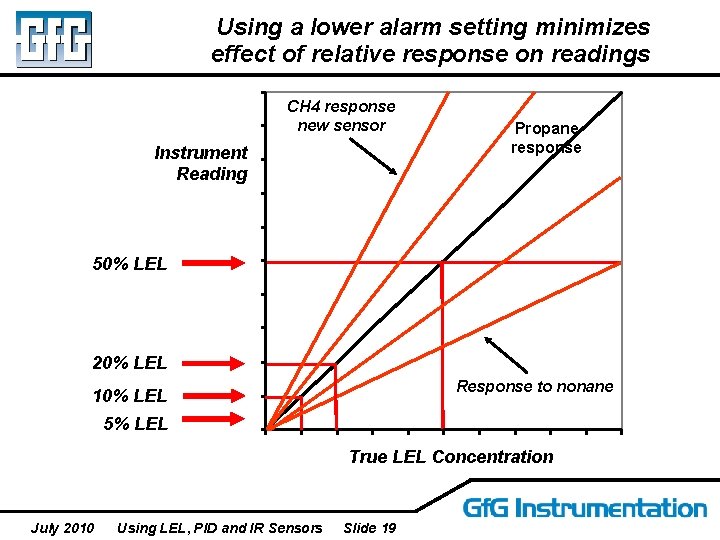
Using a lower alarm setting minimizes effect of relative response on readings CH 4 response new sensor Instrument Reading Propane response 50% LEL 20% LEL Response to nonane 10% LEL 5% LEL True LEL Concentration July 2010 Using LEL, PID and IR Sensors Slide 19

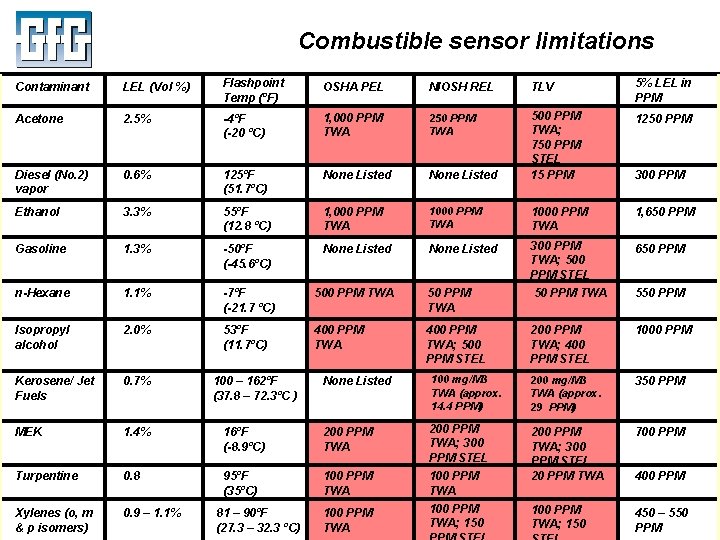
Combustible sensor limitations Contaminant LEL (Vol %) Flashpoint Temp (ºF) OSHA PEL NIOSH REL TLV 5% LEL in PPM Acetone 2. 5% -4ºF (-20 ºC) 1, 000 PPM TWA 250 PPM TWA 1250 PPM Diesel (No. 2) vapor 0. 6% 125ºF (51. 7ºC) None Listed 500 PPM TWA; 750 PPM STEL 15 PPM Ethanol 3. 3% 55ºF (12. 8 ºC) 1, 000 PPM TWA 1000 PPM TWA 1, 650 PPM Gasoline 1. 3% -50ºF (-45. 6ºC) None Listed 300 PPM TWA; 500 PPM STEL 650 PPM n-Hexane 1. 1% -7ºF (-21. 7 ºC) 500 PPM TWA 50 PPM TWA 550 PPM Isopropyl alcohol 2. 0% 53ºF (11. 7ºC) 400 PPM TWA; 500 PPM STEL 200 PPM TWA; 400 PPM STEL 1000 PPM Kerosene/ Jet Fuels 0. 7% MEK 1. 4% Turpentine 0. 8 Xylenes (o, m & p. July isomers) 2010 0. 9 – 1. 1% 300 PPM None Listed 100 mg/M 3 TWA (approx. 14. 4 PPM) 200 mg/M 3 TWA (approx. 29 PPM) 350 PPM 16ºF (-8. 9ºC) 200 PPM TWA; 300 PPM STEL 700 PPM 95ºF (35ºC) 100 PPM TWA 200 PPM TWA; 300 PPM STEL 20 PPM TWA 100 PPM TWA; 150 450 – 550 PPM 100 – 162ºF (37. 8 – 72. 3ºC ) 81 – 90ºF 100 PPM (27. 3 – 32. 3 ºC) Using LEL, PID and IR Sensors. TWA Slide 20 100 PPM TWA; 150 400 PPM

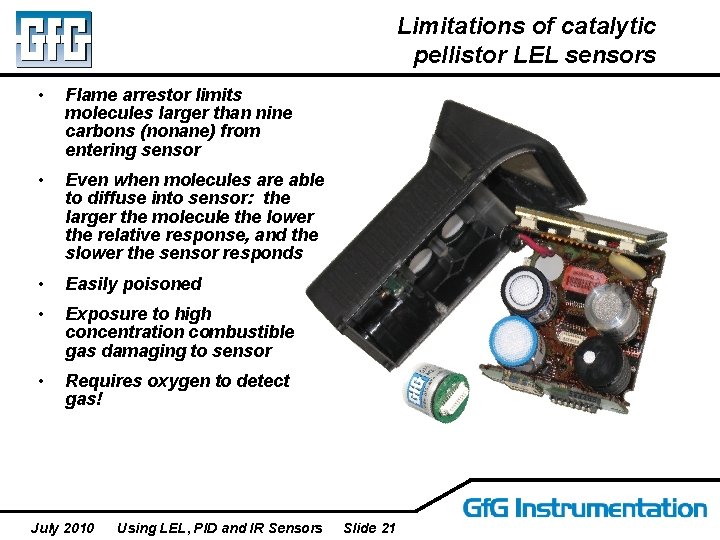
Limitations of catalytic pellistor LEL sensors • Flame arrestor limits molecules larger than nine carbons (nonane) from entering sensor • Even when molecules are able to diffuse into sensor: the larger the molecule the lower the relative response, and the slower the sensor responds • Easily poisoned • Exposure to high concentration combustible gas damaging to sensor • Requires oxygen to detect gas! July 2010 Using LEL, PID and IR Sensors Slide 21

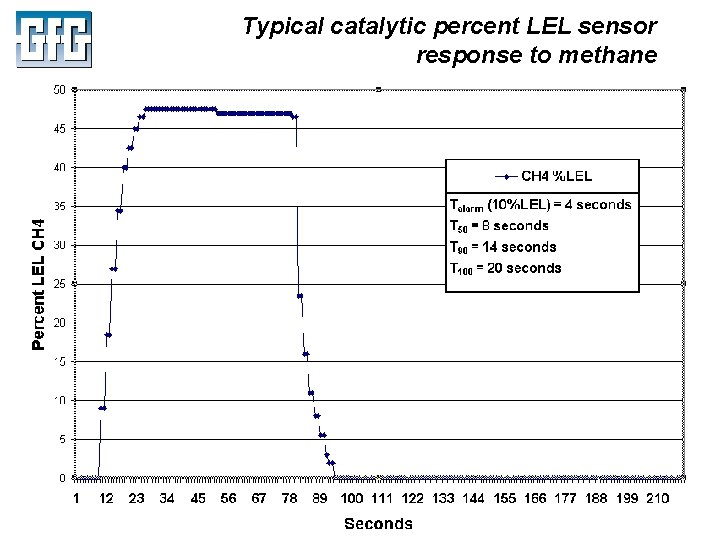
Typical catalytic percent LEL sensor response to methane July 2010 Using LEL, PID and IR Sensors Slide 22

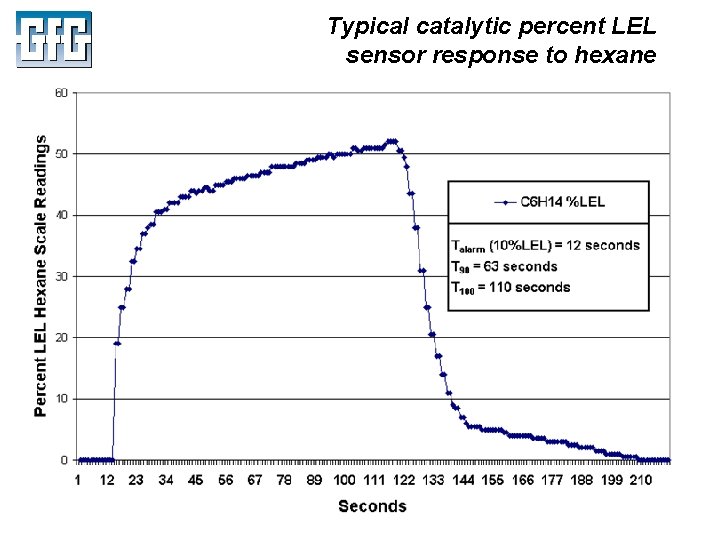
Typical catalytic percent LEL sensor response to hexane July 2010 Using LEL, PID and IR Sensors Slide 23

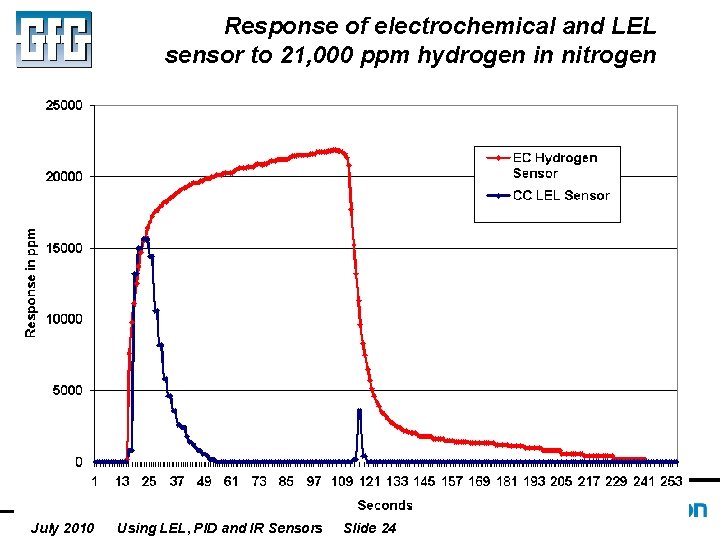
Response of electrochemical and LEL sensor to 21, 000 ppm hydrogen in nitrogen July 2010 Using LEL, PID and IR Sensors Slide 24

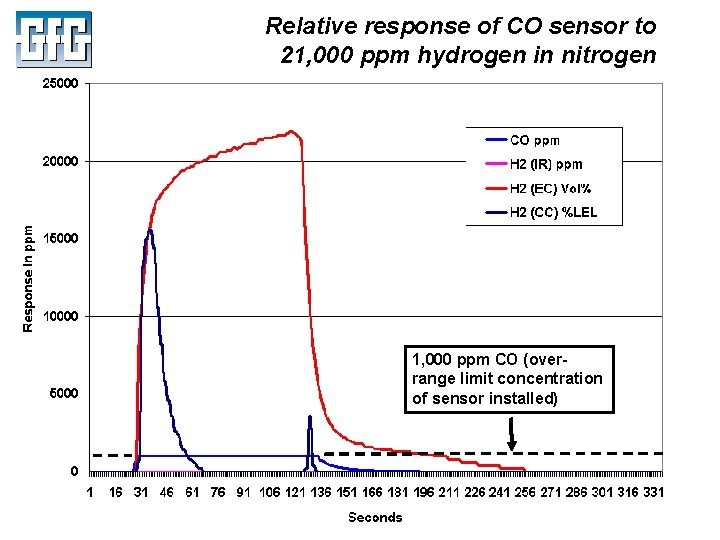
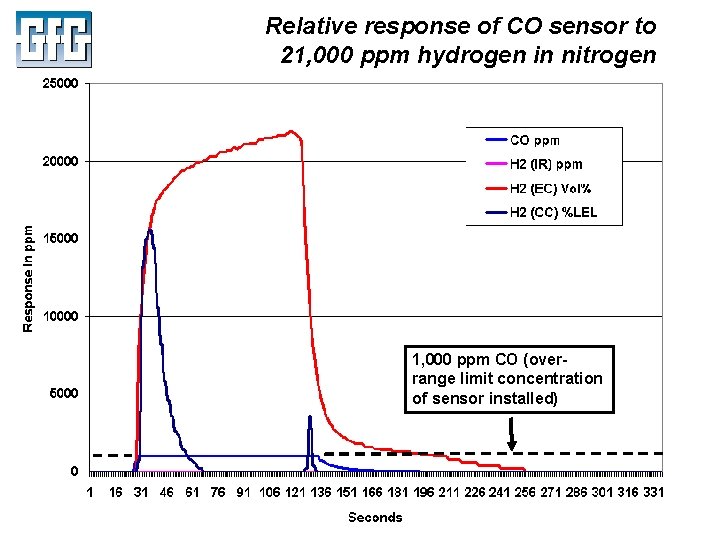
Relative response of CO sensor to 21, 000 ppm hydrogen in nitrogen 1, 000 ppm CO (overrange limit concentration of sensor installed) July 2010 Using LEL, PID and IR Sensors Slide 25

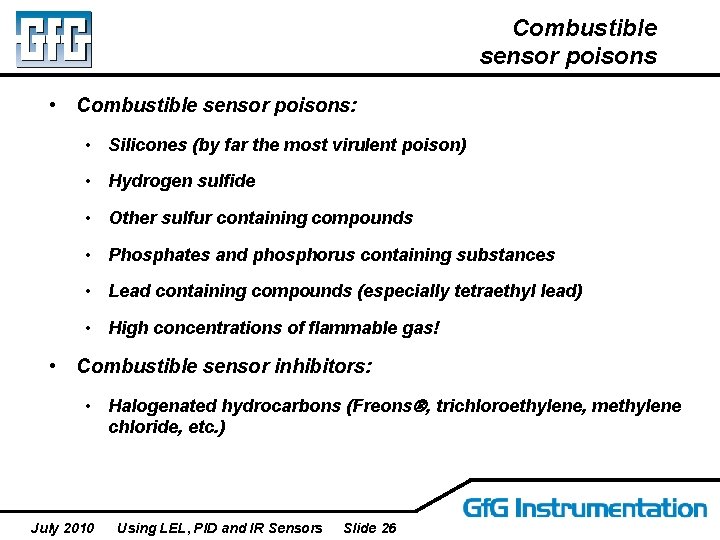
Combustible sensor poisons • Combustible sensor poisons: • Silicones (by far the most virulent poison) • Hydrogen sulfide • Other sulfur containing compounds • Phosphates and phosphorus containing substances • Lead containing compounds (especially tetraethyl lead) • High concentrations of flammable gas! • Combustible sensor inhibitors: • Halogenated hydrocarbons (Freons , trichloroethylene, methylene chloride, etc. ) July 2010 Using LEL, PID and IR Sensors Slide 26

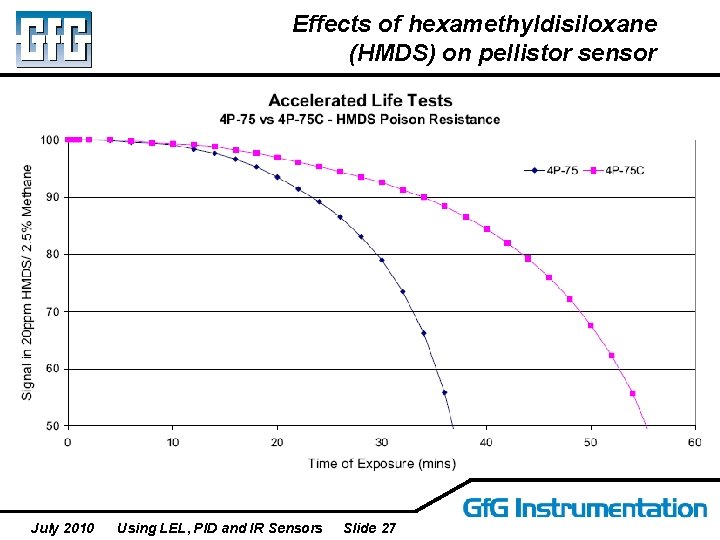
Effects of hexamethyldisiloxane (HMDS) on pellistor sensor July 2010 Using LEL, PID and IR Sensors Slide 27

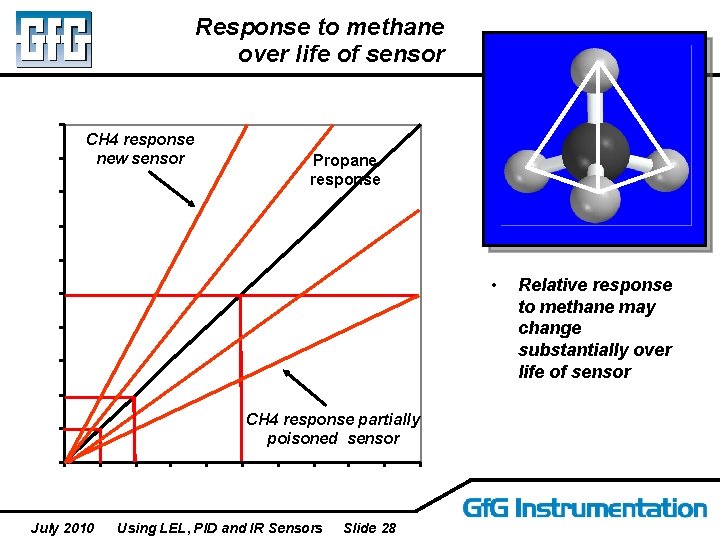
Response to methane over life of sensor CH 4 response new sensor Propane response • CH 4 response partially poisoned sensor July 2010 Using LEL, PID and IR Sensors Slide 28 Relative response to methane may change substantially over life of sensor

Non-dispersive infrared (NDIR) sensors • Many gases absorb infrared light at a unique wavelength (color) • In NDIR sensors the amount of IR light absorbed is proportional to the amount of target gas present • The longer the optical path through the semnsor the better the resolution July 2010 Using LEL, PID and IR Sensors Slide 29

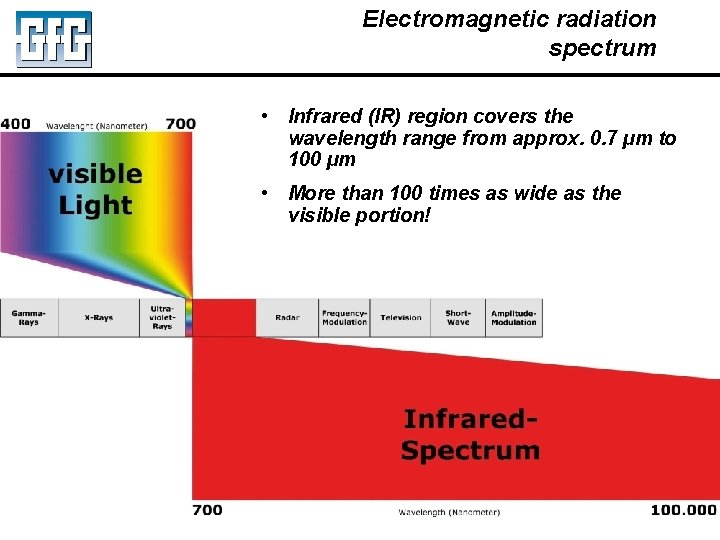
Electromagnetic radiation spectrum • Infrared (IR) region covers the wavelength range from approx. 0. 7 µm to 100 µm • More than 100 times as wide as the visible portion! July 2010 Using LEL, PID and IR Sensors Slide 30

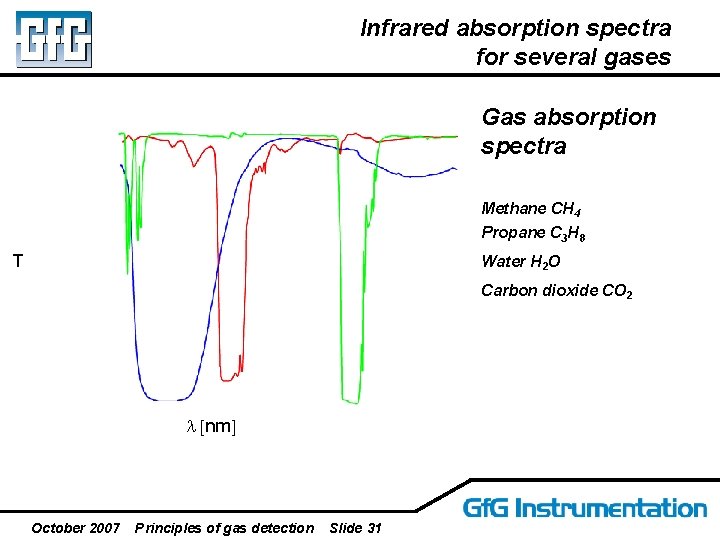
Infrared absorption spectra for several gases Gas absorption spectra Methane CH 4 Propane C 3 H 8 T Water H 2 O Carbon dioxide CO 2 l [nm] October 2007 Principles of gas detection Slide 31

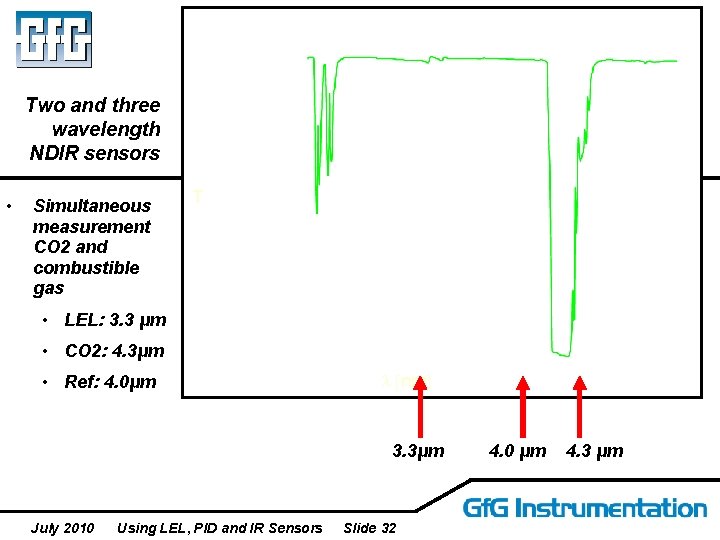
Two and three wavelength NDIR sensors • Simultaneous measurement CO 2 and combustible gas T • LEL: 3. 3 μm • CO 2: 4. 3μm • Ref: 4. 0μm l [nm] 3. 3μm July 2010 Using LEL, PID and IR Sensors Slide 32 4. 0 μm 4. 3 μm

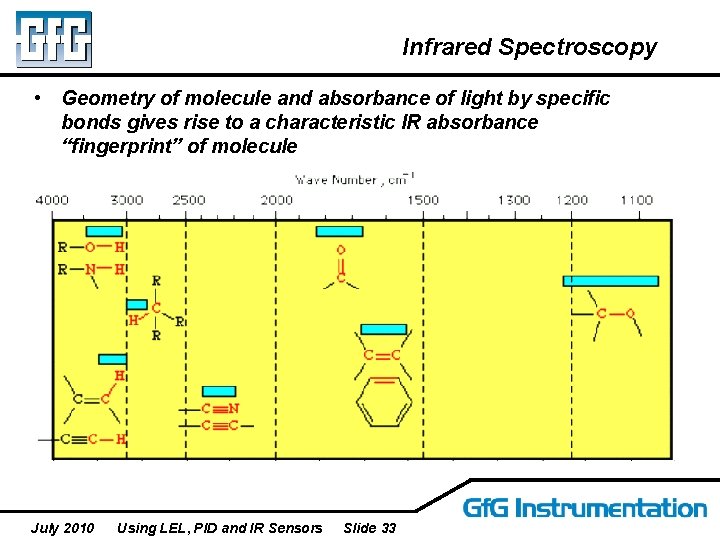
Infrared Spectroscopy • Geometry of molecule and absorbance of light by specific bonds gives rise to a characteristic IR absorbance “fingerprint” of molecule July 2010 Using LEL, PID and IR Sensors Slide 33

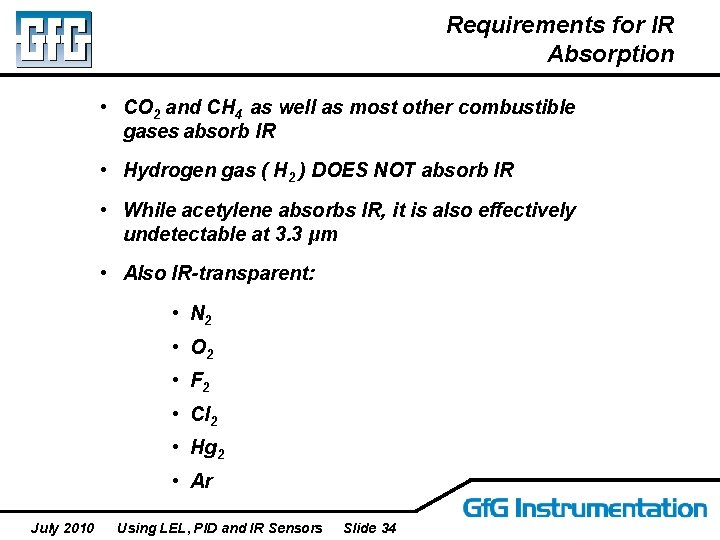
Requirements for IR Absorption • CO 2 and CH 4 as well as most other combustible gases absorb IR • Hydrogen gas ( H 2 ) DOES NOT absorb IR • While acetylene absorbs IR, it is also effectively undetectable at 3. 3 μm • Also IR-transparent: • N 2 • O 2 • F 2 • Cl 2 • Hg 2 • Ar July 2010 Using LEL, PID and IR Sensors Slide 34

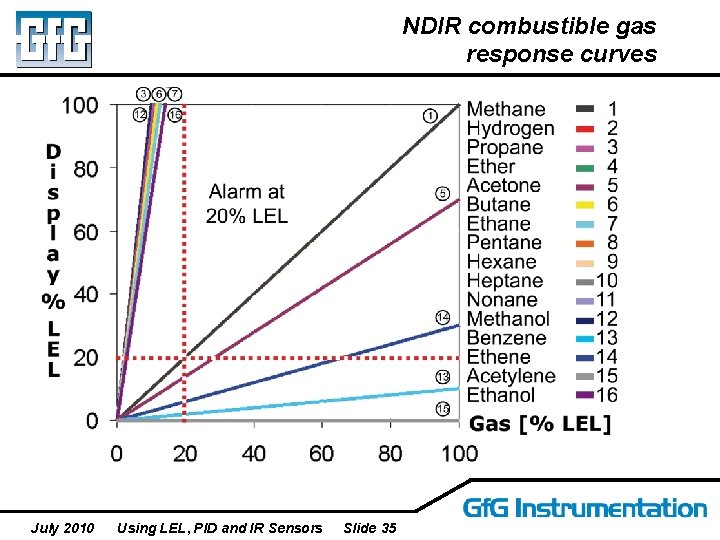
NDIR combustible gas response curves July 2010 Using LEL, PID and IR Sensors Slide 35

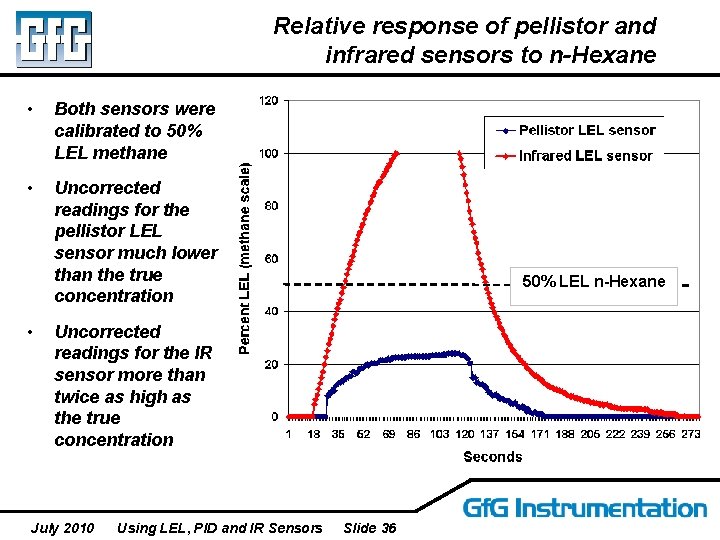
Relative response of pellistor and infrared sensors to n-Hexane • Both sensors were calibrated to 50% LEL methane • Uncorrected readings for the pellistor LEL sensor much lower than the true concentration • 50% LEL n-Hexane Uncorrected readings for the IR sensor more than twice as high as the true concentration July 2010 Using LEL, PID and IR Sensors Slide 36

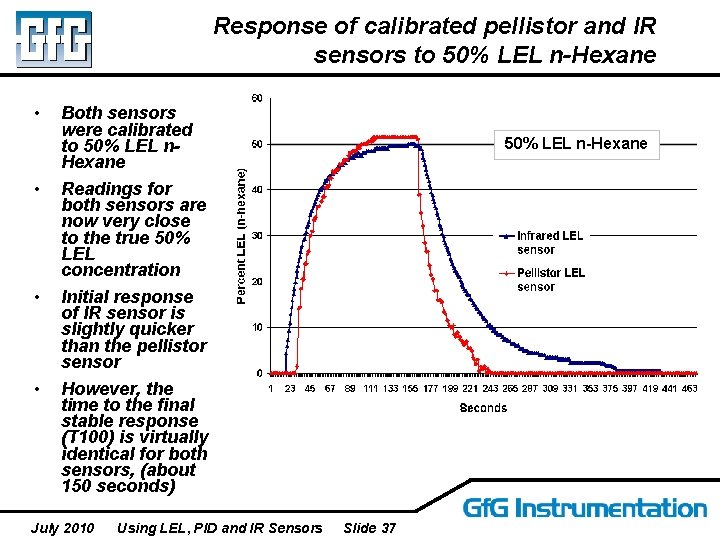
Response of calibrated pellistor and IR sensors to 50% LEL n-Hexane • Both sensors were calibrated to 50% LEL n. Hexane • Readings for both sensors are now very close to the true 50% LEL concentration • Initial response of IR sensor is slightly quicker than the pellistor sensor However, the time to the final stable response (T 100) is virtually identical for both sensors, (about 150 seconds) • July 2010 Using LEL, PID and IR Sensors 50% LEL n-Hexane Slide 37

Relative response of CO sensor to 21, 000 ppm hydrogen in nitrogen 1, 000 ppm CO (overrange limit concentration of sensor installed) July 2010 Using LEL, PID and IR Sensors Slide 38

Photoionization Detectors • Used for measuring solvent, fuel and VOC vapors in the workplace environment July 2010 Using LEL, PID and IR Sensors Slide 39

LEL vs. PID Sensors • Catalytic LEL and PID sensors are complementary detection techniques • Catalytic LEL sensors excellent for methane, propane, and other common combustible gases that are NOT detectable by PID • PIDs detect large VOC and hydrocarbon molecules that are undetectable by hotbead sensors • Best approach is to use multi-sensor instrument that includes both types of sensors July 2010 Using LEL, PID and IR Sensors Slide 40

Choosing the best sensor configuration • Multi-sensor instruments can include up to seven channels of real-time measurement • Available sensors for combustible gas and VOC measurement: : • CC %LEL • IR %Vol • Thermal Conductivity %Vol • Electrochemical toxic • PID July 2010 Using LEL, PID and IR Sensors Slide 41

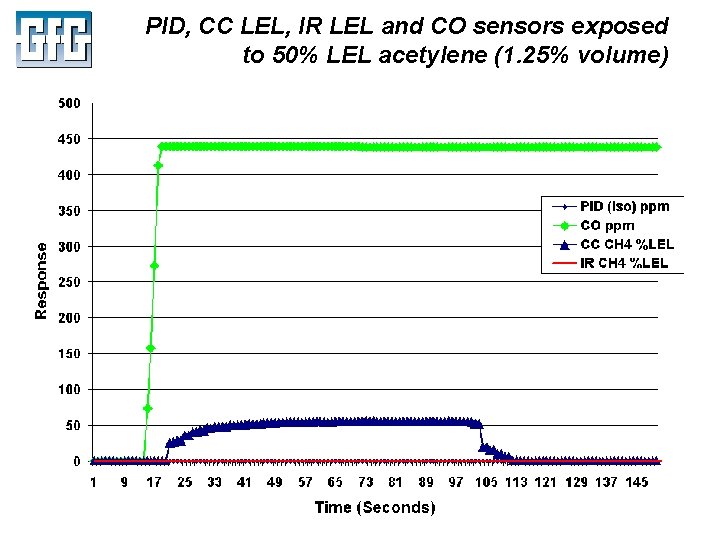
PID, CC LEL, IR LEL and CO sensors exposed to 50% LEL acetylene (1. 25% volume) July 2010 Using LEL, PID and IR Sensors Slide 42

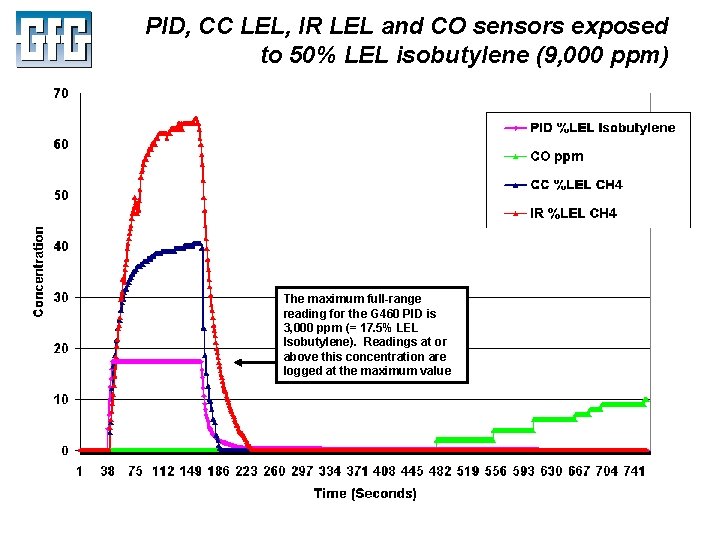
PID, CC LEL, IR LEL and CO sensors exposed to 50% LEL isobutylene (9, 000 ppm) The maximum full-range reading for the G 460 PID is 3, 000 ppm (= 17. 5% LEL Isobutylene). Readings at or above this concentration are logged at the maximum value July 2010 Using LEL, PID and IR Sensors Slide 43

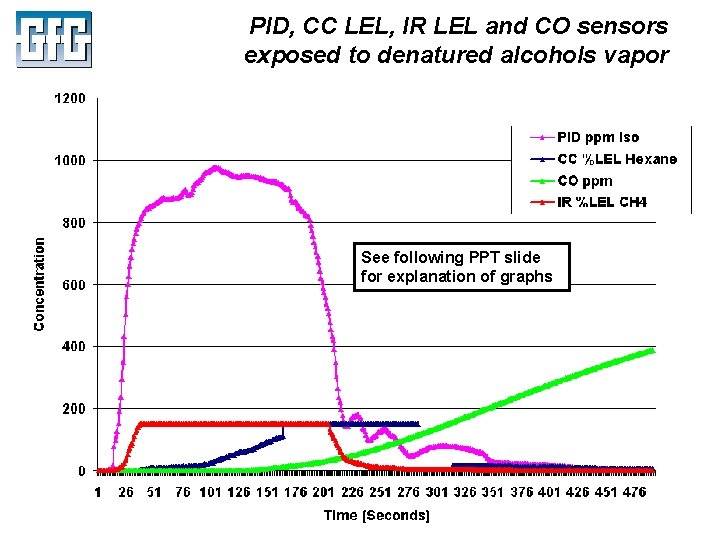
PID, CC LEL, IR LEL and CO sensors exposed to denatured alcohols vapor See following PPT slide for explanation of graphs July 2010 Using LEL, PID and IR Sensors Slide 44

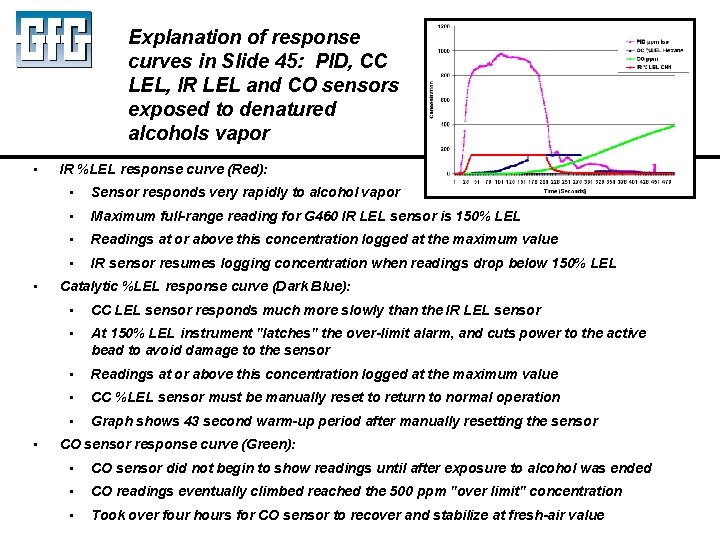
Explanation of response curves in Slide 45: PID, CC LEL, IR LEL and CO sensors exposed to denatured alcohols vapor • • • IR %LEL response curve (Red): • Sensor responds very rapidly to alcohol vapor • Maximum full-range reading for G 460 IR LEL sensor is 150% LEL • Readings at or above this concentration logged at the maximum value • IR sensor resumes logging concentration when readings drop below 150% LEL Catalytic %LEL response curve (Dark Blue): • CC LEL sensor responds much more slowly than the IR LEL sensor • At 150% LEL instrument "latches" the over-limit alarm, and cuts power to the active bead to avoid damage to the sensor • Readings at or above this concentration logged at the maximum value • CC %LEL sensor must be manually reset to return to normal operation • Graph shows 43 second warm-up period after manually resetting the sensor CO sensor response curve (Green): • CO sensor did not begin to show readings until after exposure to alcohol was ended • CO readings eventually climbed reached the 500 ppm "over limit" concentration • Took over four hours for CO sensor to recover and stabilize at fresh-air value July 2010 Using LEL, PID and IR Sensors Slide 45

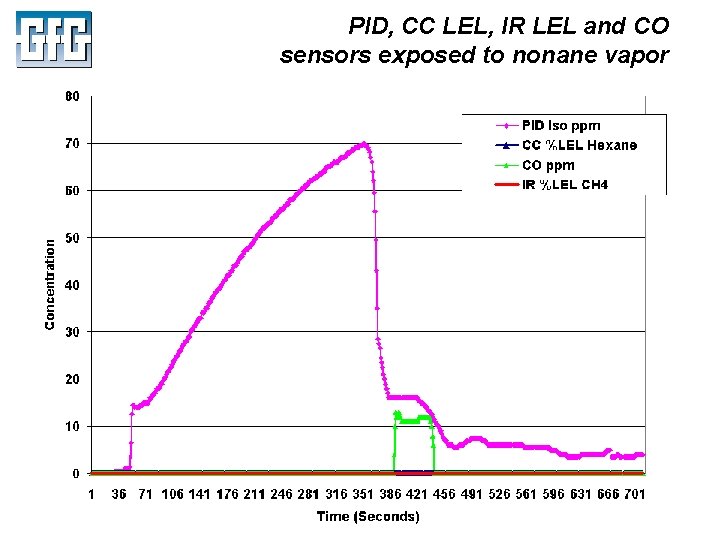
PID, CC LEL, IR LEL and CO sensors exposed to nonane vapor July 2010 Using LEL, PID and IR Sensors Slide 46

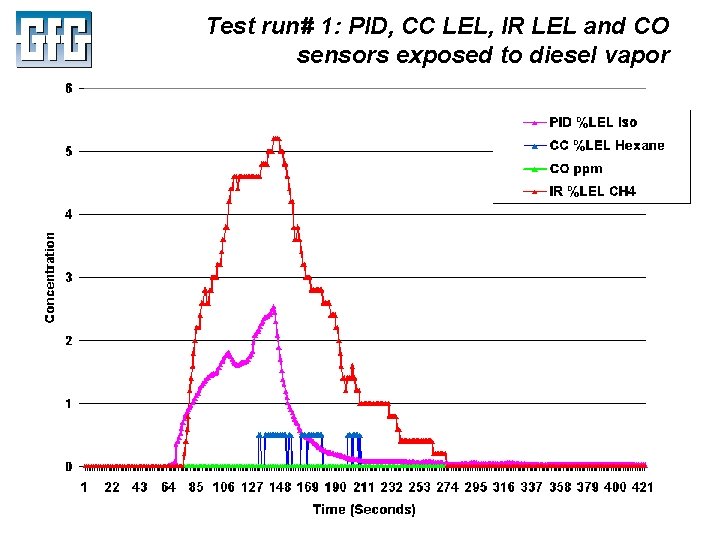
Test run# 1: PID, CC LEL, IR LEL and CO sensors exposed to diesel vapor July 2010 Using LEL, PID and IR Sensors Slide 47

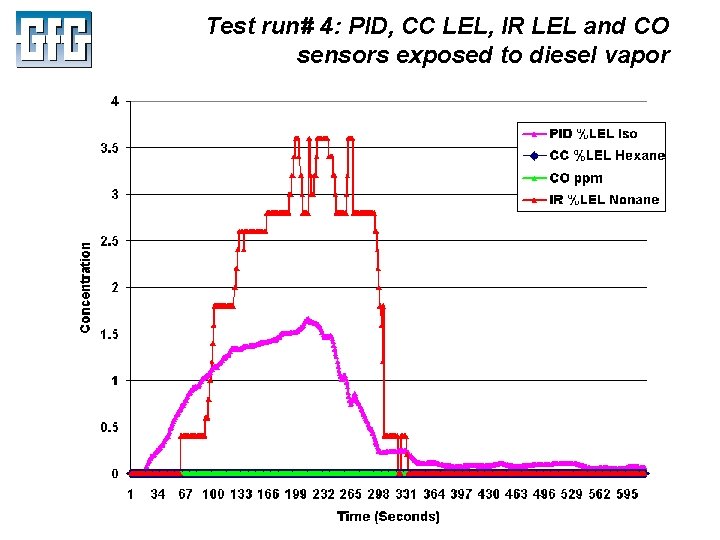
Test run# 4: PID, CC LEL, IR LEL and CO sensors exposed to diesel vapor July 2010 Using LEL, PID and IR Sensors Slide 48

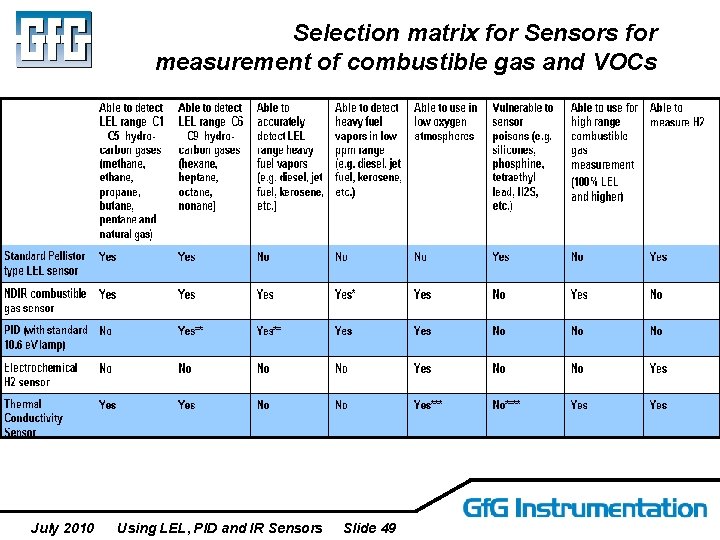
Selection matrix for Sensors for measurement of combustible gas and VOCs July 2010 Using LEL, PID and IR Sensors Slide 49

Questions? July 2010 Using LEL, PID and IR Sensors Slide 50