Choosing a combination OCP Comparing risks of PE

Choosing a combination OCP: Comparing risks of PE, MI, & CVA Andrea Dotson Lauren Bauer August 23, 2017

Schedule for Today • • • 5 min - Introduction, background 35 min - Group work 10 min - Reporting from groups 5 min - EBM pearl 5 min - Questions, comments 1 min - Evaluation

“Mona” • Your patient is a 36 year old female with an unremarkable medical history who presents to discuss birth control with combination (estrogen/progestin) contraceptive pills (COC). She has no previous history of HTN, DM, Cancer, CAD, CVA, or PE. She does not smoke tobacco. • She read about a case in France of a woman who had a stroke after starting a 3 rd generation OCP with gestodene (GSD) and ethinyl-estradiol (EE). She wants you to prescribe a COC with the lowest risk of causing CVA, MI, or PE.

Background • “In December 2012, a young French woman sued a drug company and the head of the French drug regulatory Agency (ANSM) after she had a stroke with major sequellae when using a thirdgeneration pill containing gestodene (GSD) and ethinyl-estradiol (EE). This case, which received extensive media coverage, has provoked alarm in France (1, 2). Subsequently, contraceptive practices have changed. This case highlighted the risks of vascular thromboembolic diseases associated with pills and, in particular, with the use of newer pills. ”

Epidemiology • Combined oral contraceptive pills (COCs) are the most common form of reversible birth control in developing countries – Over 100 million women worldwide • COCs are known to increase coagulation factors, triglycerides, LDL, insulin, and decrease glucose tolerance all known risk factors for CVD • Cochrane Review showed 1. 6 increased risk of arterial thrombosis among women using COCs compared to those who did not
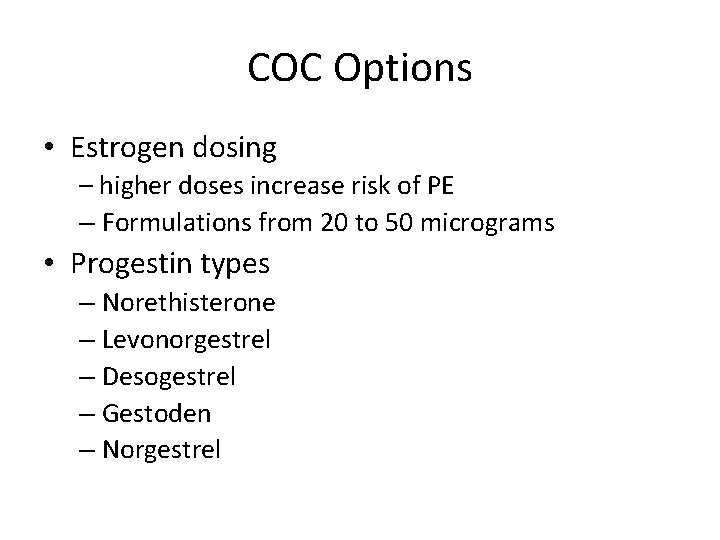
COC Options • Estrogen dosing – higher doses increase risk of PE – Formulations from 20 to 50 micrograms • Progestin types – Norethisterone – Levonorgestrel – Desogestrel – Gestoden – Norgestrel

Objectives • Formulate a clinically relevant question and identify population, exposure, comparison, outcome, and type of study (PICOT) to address patient question • Find research study to address this question (thank you AFP May 1, 2017) • What are the results of the study • Are the results valid • Apply these results to your patient

Now… • • • Materials distributed Split into 3 groups Formulate PICOT (5 minutes) Review abstract, methods, results (10 min) Complete critical appraisal (20 min) Report (10 min) EBM pearl (5 min) Questions (5 min) Complete evaluation (1 min)
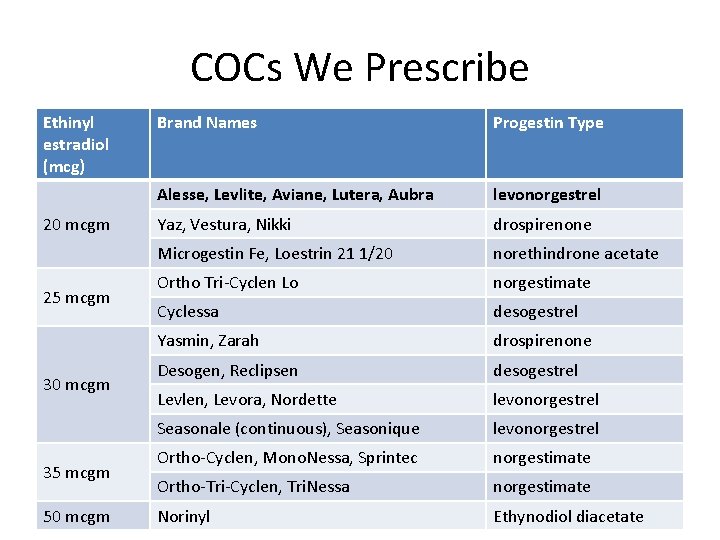
COCs We Prescribe Ethinyl estradiol (mcg) 20 mcgm 25 mcgm 30 mcgm 35 mcgm 50 mcgm Brand Names Progestin Type Alesse, Levlite, Aviane, Lutera, Aubra levonorgestrel Yaz, Vestura, Nikki drospirenone Microgestin Fe, Loestrin 21 1/20 norethindrone acetate Ortho Tri-Cyclen Lo norgestimate Cyclessa desogestrel Yasmin, Zarah drospirenone Desogen, Reclipsen desogestrel Levlen, Levora, Nordette levonorgestrel Seasonale (continuous), Seasonique levonorgestrel Ortho-Cyclen, Mono. Nessa, Sprintec norgestimate Ortho-Tri-Cyclen, Tri. Nessa norgestimate Norinyl Ethynodiol diacetate

EBM Pearl: OR or RR • Relative risk (RR) is the probability that a member of an exposed group will develop a disease relative to the probability that a member of an unexposed group will develop that same disease • Odds ratio (OR) is the odds of disease among exposed individuals divided by the odds of disease among unexposed – Remember: “Odds” is the probability that the event WILL occur to the probability that the event will NOT occur
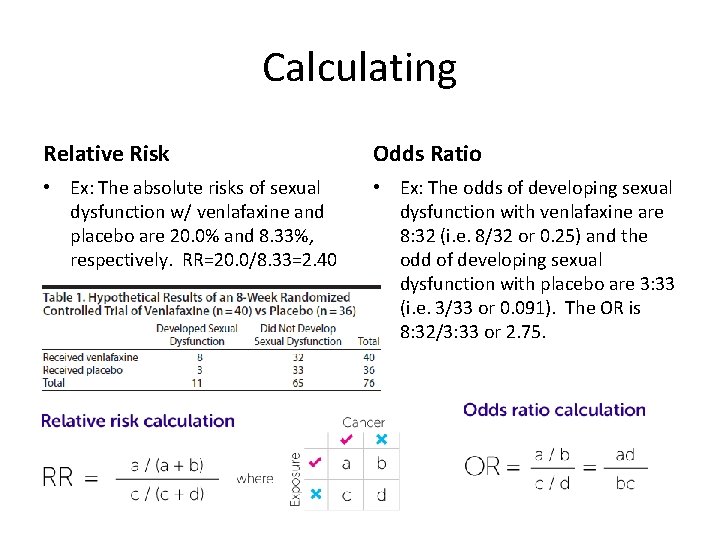
Calculating Relative Risk Odds Ratio • Ex: The absolute risks of sexual dysfunction w/ venlafaxine and placebo are 20. 0% and 8. 33%, respectively. RR=20. 0/8. 33=2. 40 • Ex: The odds of developing sexual dysfunction with venlafaxine are 8: 32 (i. e. 8/32 or 0. 25) and the odd of developing sexual dysfunction with placebo are 3: 33 (i. e. 3/33 or 0. 091). The OR is 8: 32/3: 33 or 2. 75.

Interpreting OR and RR • RR or OR = 1 – Risk in exposed = risk in non-exposed (RR) – Exposure is not related to disease (OR) – No association • RR or OR >1 – Risk in exposed > risk in non-exposed (RR) – Exposure is positively related to disease (OR) – Positive association, ? causal • RR or OR <1 – Risk in exposed < risk in non-exposed (RR) – Exposure is negatively related to disease (OR) – Negative association, ? protective
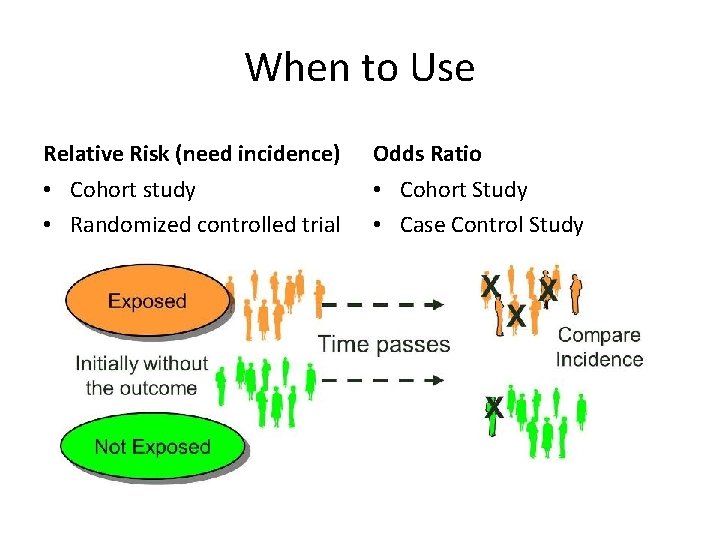
When to Use Relative Risk (need incidence) Odds Ratio • Cohort study • Randomized controlled trial • Cohort Study • Case Control Study

Questions?
- Slides: 14