Choose Your Category The Mole Average Atomic Mass




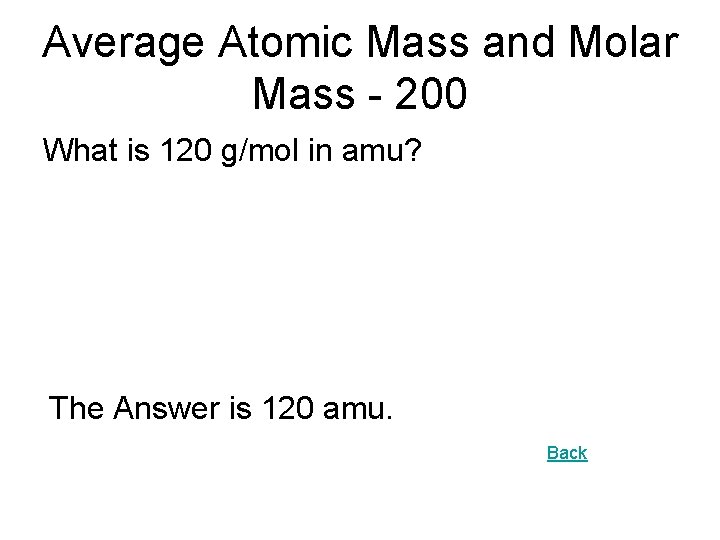
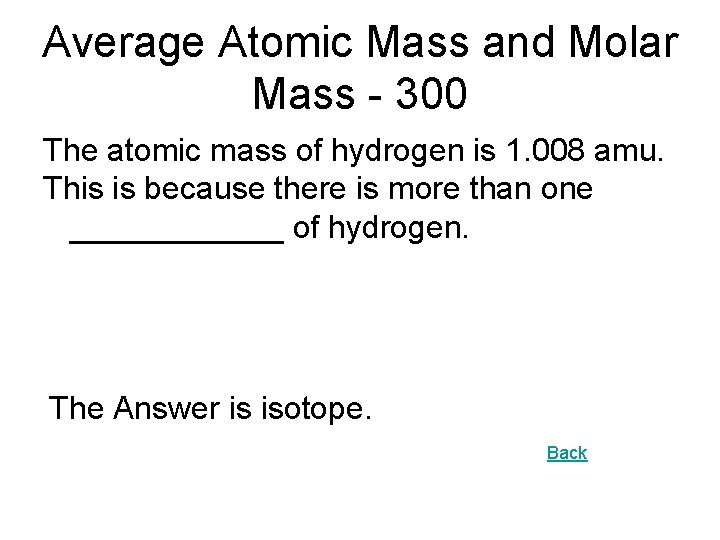




















- Slides: 46

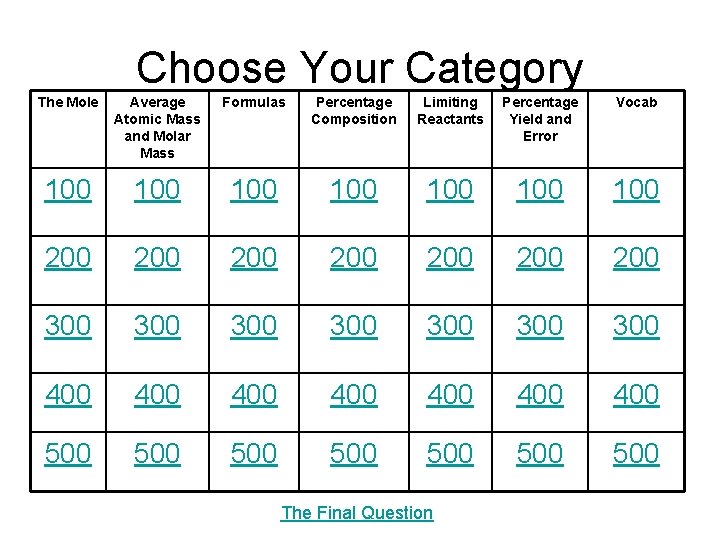
Choose Your Category The Mole Average Atomic Mass and Molar Mass Formulas Percentage Composition Limiting Reactants Percentage Yield and Error Vocab 100 100 200 200 300 300 400 400 500 500 The Final Question

The Mole - 100 • The SI base unit used to measure the amount of a substance whose number of particles equals the number of atoms of carbon in exactly 12 grams of carbon-12 The Answer is the mole Back

The Mole - 200 • The number of atoms in a mole of any pure substance is called The Answer is the Avogadro's number Back

The Mole - 300 • What can be said about the atoms in 1 mol Ag and 1 mol Au? The Answer is they contain the same number of atoms. Back

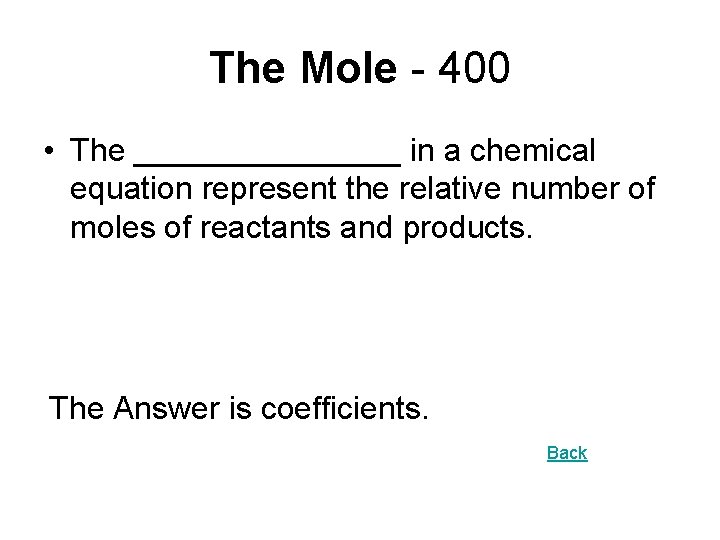
The Mole - 400 • The ________ in a chemical equation represent the relative number of moles of reactants and products. The Answer is coefficients. Back

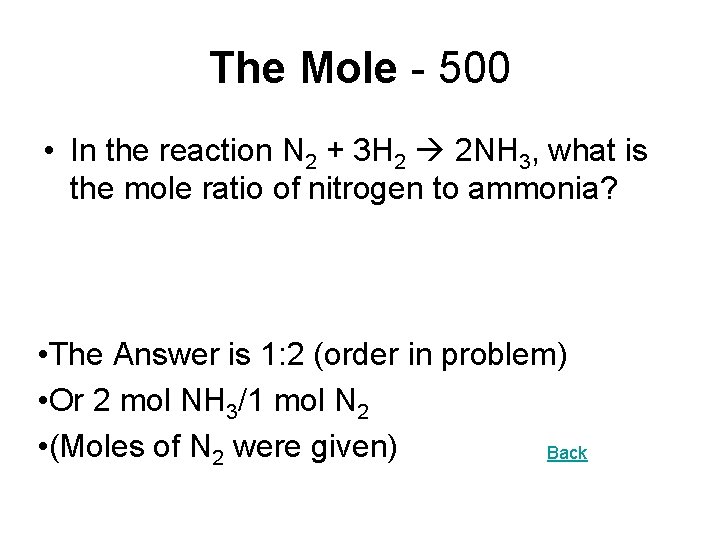
The Mole - 500 • In the reaction N 2 + 3 H 2 2 NH 3, what is the mole ratio of nitrogen to ammonia? • The Answer is 1: 2 (order in problem) • Or 2 mol NH 3/1 mol N 2 • (Moles of N 2 were given) Back

Average Atomic Mass and Molar Mass - 100 • Using a periodic table, what is the average atomic mass of zinc? The Answer is 65. 4 amu. Back
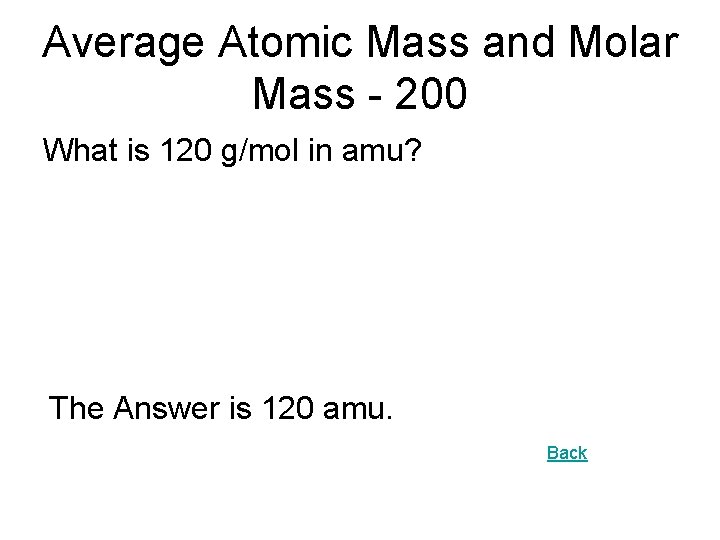
Average Atomic Mass and Molar Mass - 200 What is 120 g/mol in amu? The Answer is 120 amu. Back
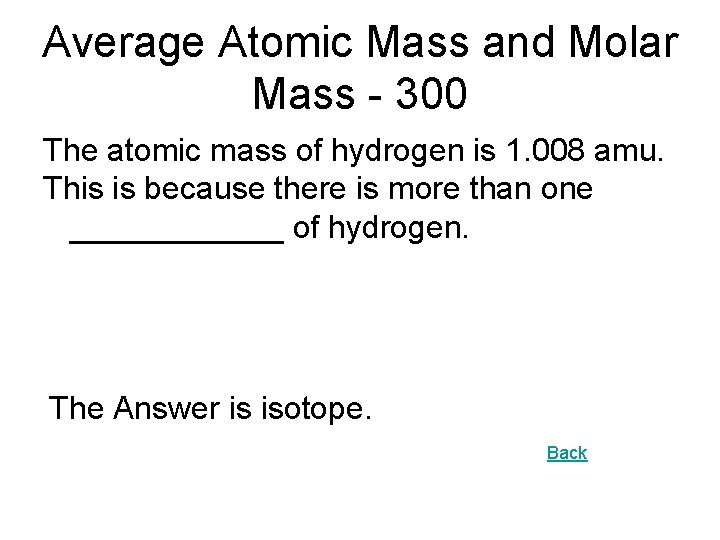
Average Atomic Mass and Molar Mass - 300 The atomic mass of hydrogen is 1. 008 amu. This is because there is more than one ______ of hydrogen. The Answer is isotope. Back

Random Points 600 points

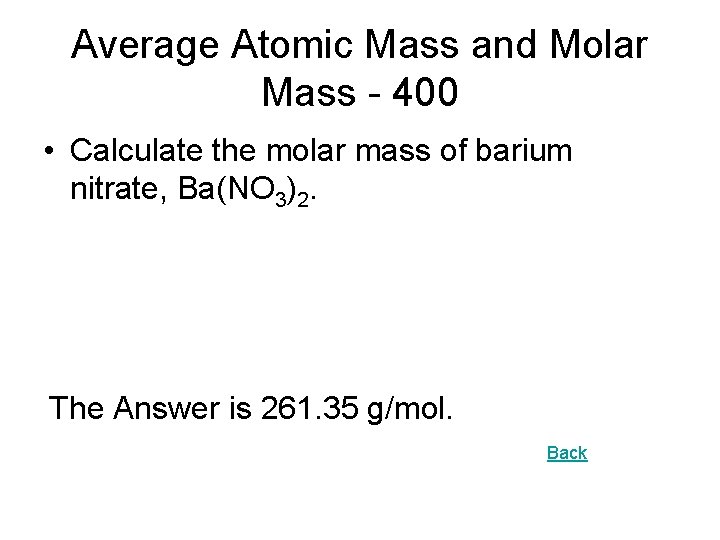
Average Atomic Mass and Molar Mass - 400 • Calculate the molar mass of barium nitrate, Ba(NO 3)2. The Answer is 261. 35 g/mol. Back

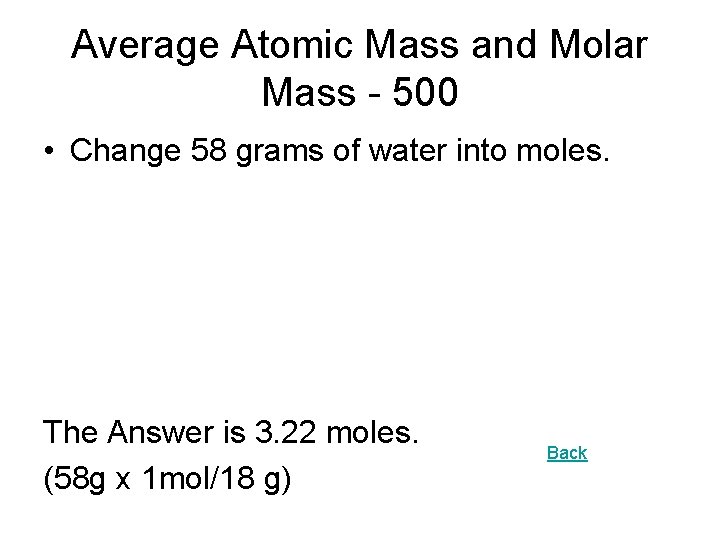
Average Atomic Mass and Molar Mass - 500 • Change 58 grams of water into moles. The Answer is 3. 22 moles. (58 g x 1 mol/18 g) Back

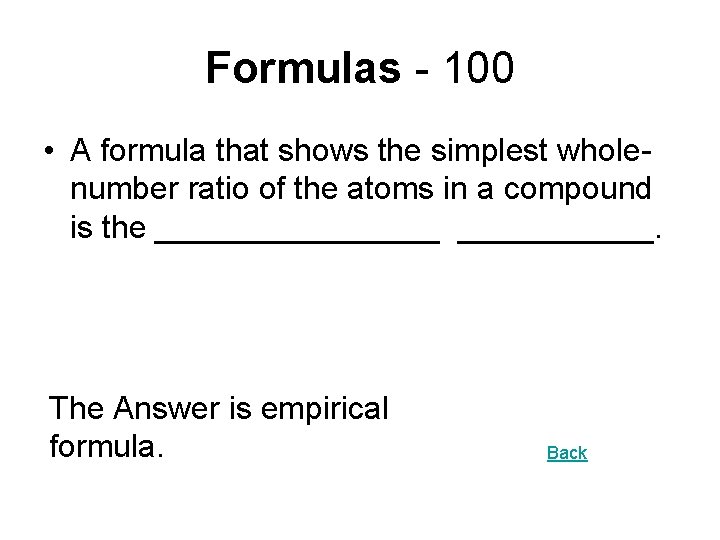
Formulas - 100 • A formula that shows the simplest wholenumber ratio of the atoms in a compound is the ________. The Answer is empirical formula. Back

Formulas - 200 • The first step in finding the empirical formula from the percentage composition is to assume that you have this many grams? • The Answer is 100 g. Back

Formulas - 300 A compound’s empirical formula is NO 2. If the formula mass is 92 amu, what is the molecular formula? The Answer is N 2 O 4 Back

Random Points 500 points

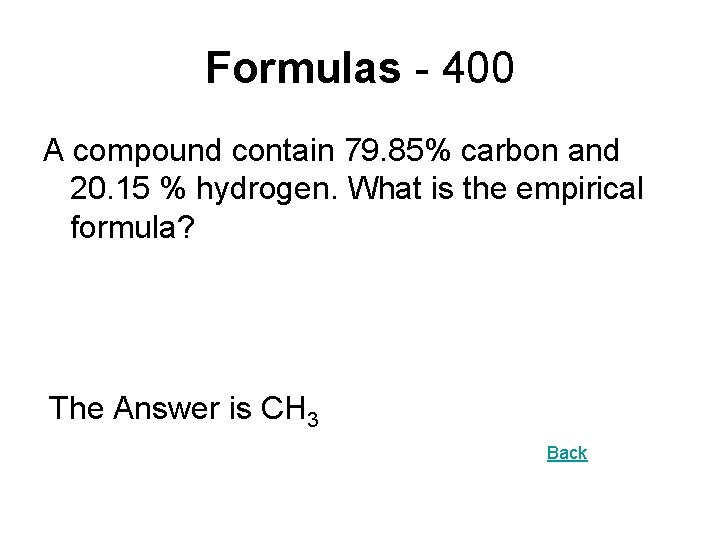
Formulas - 400 A compound contain 79. 85% carbon and 20. 15 % hydrogen. What is the empirical formula? The Answer is CH 3 Back

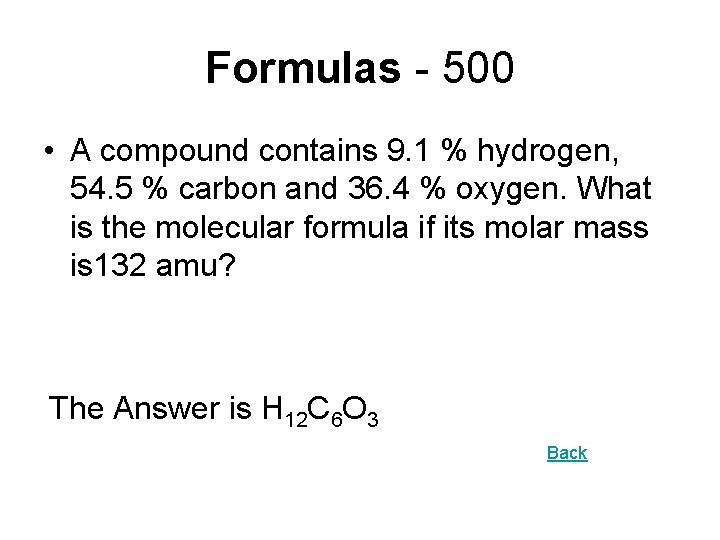
Formulas - 500 • A compound contains 9. 1 % hydrogen, 54. 5 % carbon and 36. 4 % oxygen. What is the molecular formula if its molar mass is 132 amu? The Answer is H 12 C 6 O 3 Back

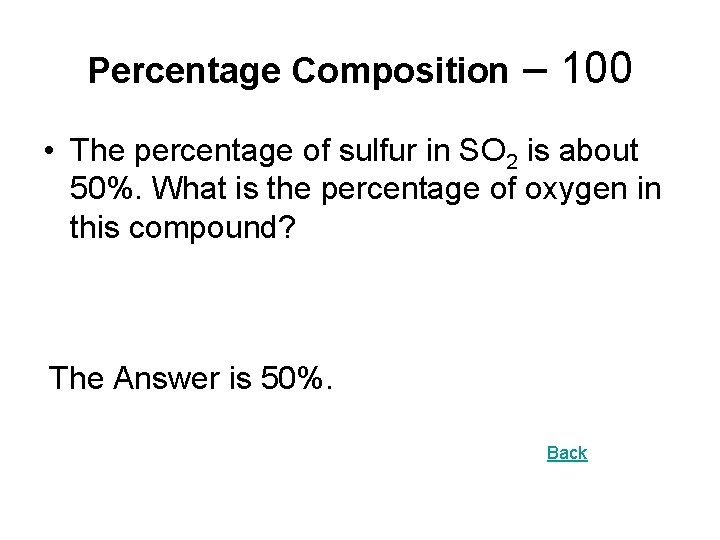
Percentage Composition – 100 • The percentage of sulfur in SO 2 is about 50%. What is the percentage of oxygen in this compound? The Answer is 50%. Back

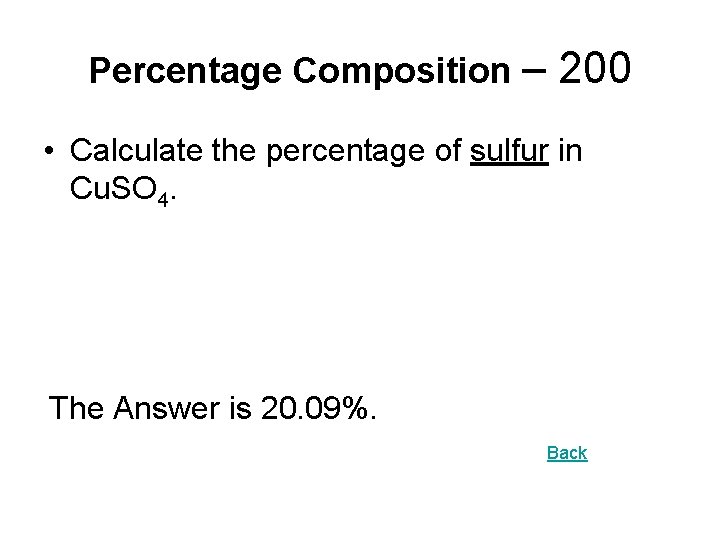
Percentage Composition – 200 • Calculate the percentage of sulfur in Cu. SO 4. The Answer is 20. 09%. Back

Random Points 300 points

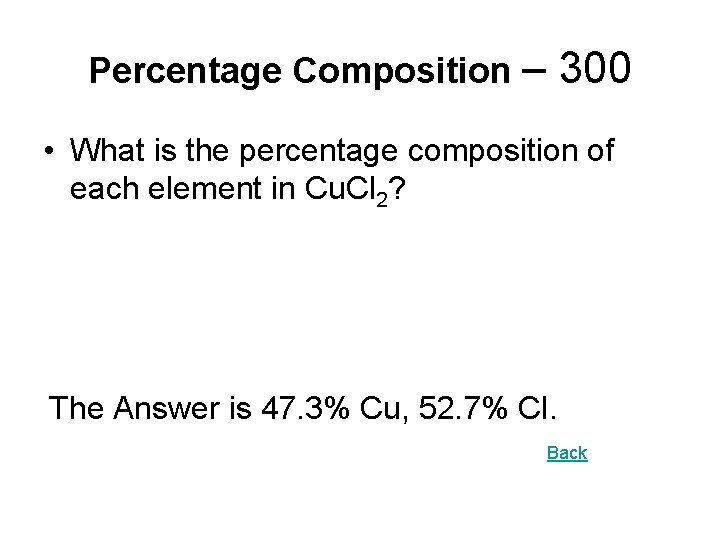
Percentage Composition – 300 • What is the percentage composition of each element in Cu. Cl 2? The Answer is 47. 3% Cu, 52. 7% Cl. Back

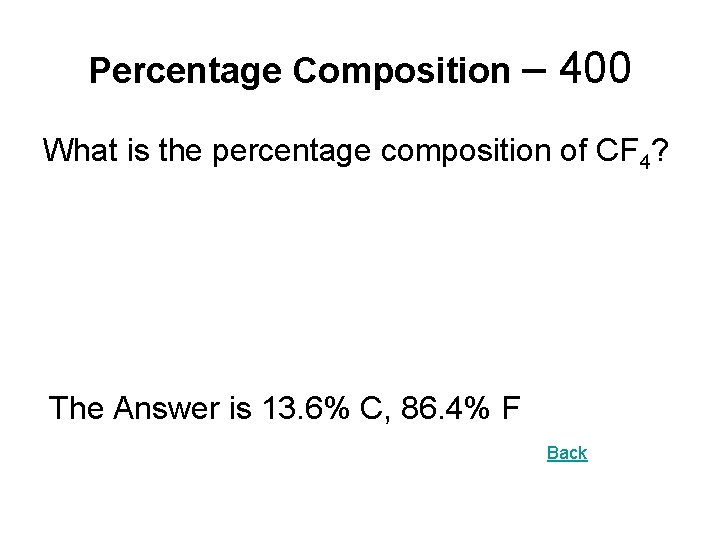
Percentage Composition – 400 What is the percentage composition of CF 4? The Answer is 13. 6% C, 86. 4% F Back

Daily Double

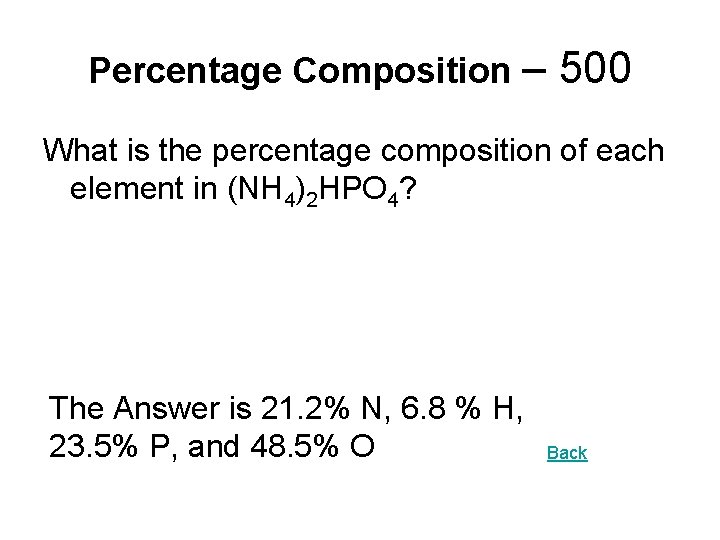
Percentage Composition – 500 What is the percentage composition of each element in (NH 4)2 HPO 4? The Answer is 21. 2% N, 6. 8 % H, 23. 5% P, and 48. 5% O Back

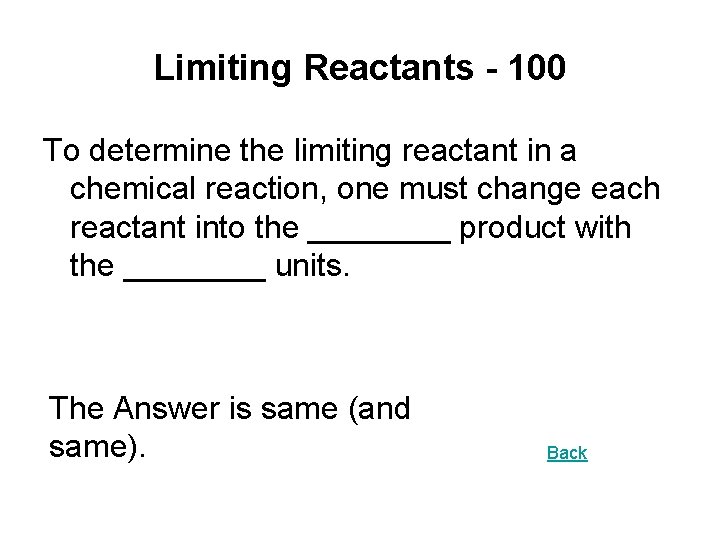
Limiting Reactants - 100 To determine the limiting reactant in a chemical reaction, one must change each reactant into the ____ product with the ____ units. The Answer is same (and same). Back

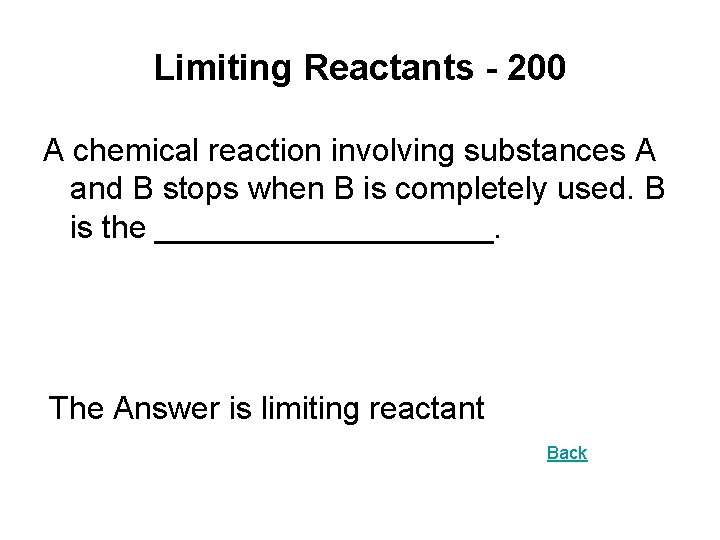
Limiting Reactants - 200 A chemical reaction involving substances A and B stops when B is completely used. B is the __________. The Answer is limiting reactant Back

Limiting Reactants - 300 All the other reactants besides the limiting reactant are called ______ reactants The Answer is excess. Back

Limiting Reactants - 400 • The first step in most stoichiometry problems is to _______. The Answer is convert given quantities to moles. Back

Random Points 800 points

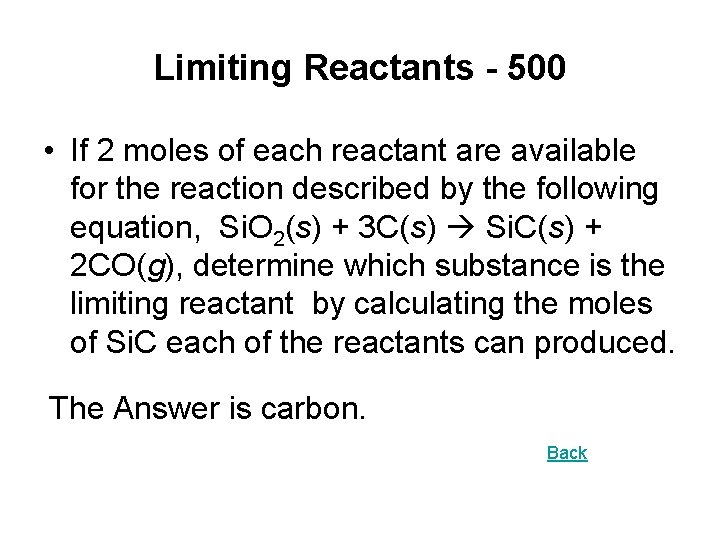
Limiting Reactants - 500 • If 2 moles of each reactant are available for the reaction described by the following equation, Si. O 2(s) + 3 C(s) Si. C(s) + 2 CO(g), determine which substance is the limiting reactant by calculating the moles of Si. C each of the reactants can produced. The Answer is carbon. Back

Percentage Yield and Error - 100 What is the ratio of the actual yield to theoretical yield, multiplied by 100%? The Answer is percentage yield Back

Percentage Yield and Error - 200 • Actual yield must be determined by _________. The Answer is experiments. Back

Random Points 400 points

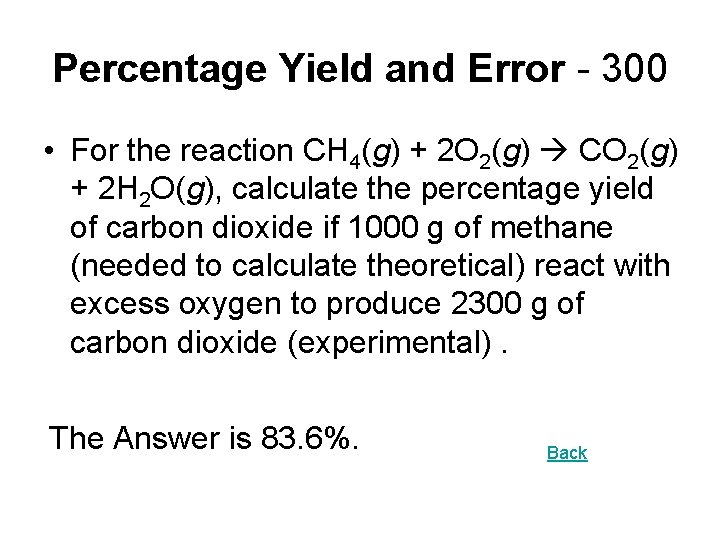
Percentage Yield and Error - 300 • For the reaction CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g), calculate the percentage yield of carbon dioxide if 1000 g of methane (needed to calculate theoretical) react with excess oxygen to produce 2300 g of carbon dioxide (experimental). The Answer is 83. 6%. Back

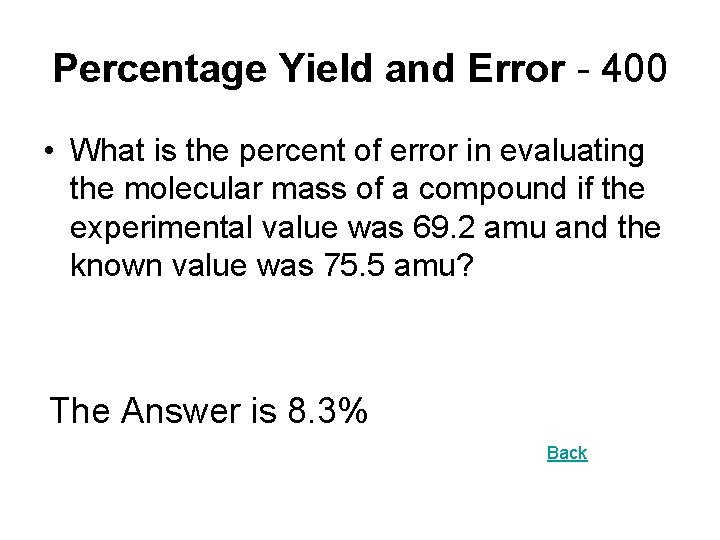
Percentage Yield and Error - 400 • What is the percent of error in evaluating the molecular mass of a compound if the experimental value was 69. 2 amu and the known value was 75. 5 amu? The Answer is 8. 3% Back

Random Points 400 points

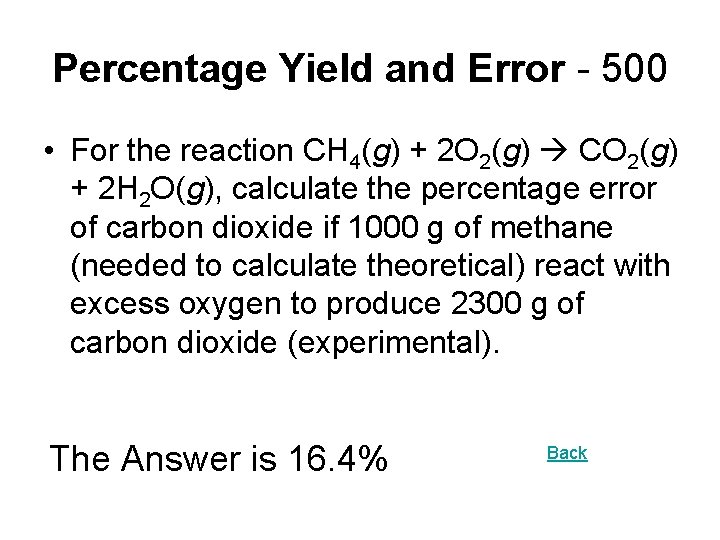
Percentage Yield and Error - 500 • For the reaction CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g), calculate the percentage error of carbon dioxide if 1000 g of methane (needed to calculate theoretical) react with excess oxygen to produce 2300 g of carbon dioxide (experimental). The Answer is 16. 4% Back

Vocab - 100 • The proportional relationship between two or more substances during a chemical reaction The Answer is stoichiometry Back

Vocab - 200 • The mass in grams of 1 mol of a substance The Answer is the molar mass Back

Vocab - 300 • The percentage by mass of each element in a compound The Answer is the percentage composition Back

Vocab - 400 • The measured amount of a product of a reaction The Answer is actual yield Back

Vocab - 500 • A chemical formula that shows the number and kinds of atoms in a molecule, but not the arrangement of the atoms The Answer is the molecular formula Back

The Final Question • The final question has to deal with: » Stoichiometry Make your wager and hand it to the teacher

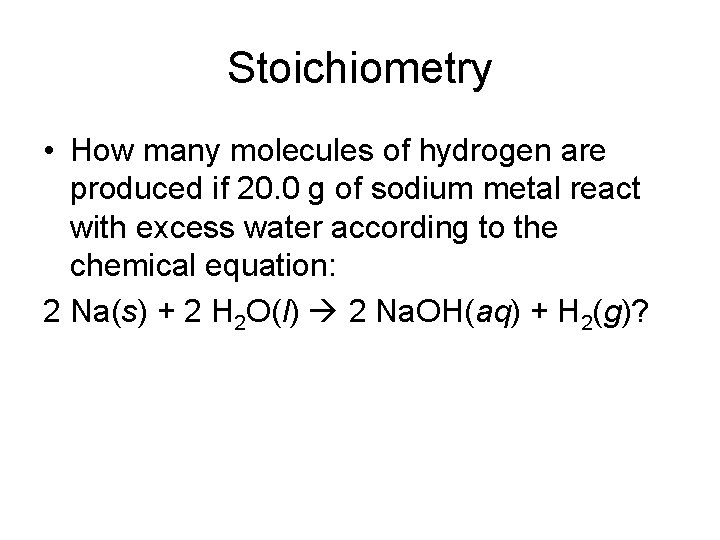
Stoichiometry • How many molecules of hydrogen are produced if 20. 0 g of sodium metal react with excess water according to the chemical equation: 2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g)?

And the Answer is: 20. 0 x (1 mol/23 g) = 0. 87 mol Na x (1 mol H 2/2 mol Na) = 0. 435 mol H 2 x (6. 02 x 1023 molecules/1 mol) = 23 The Answer is 2. 62 x 10