Choose a category You will be given the

Choose a category. You will be given the answer. You must give the correct question. Click to begin.

Click here for Final Jeopardy
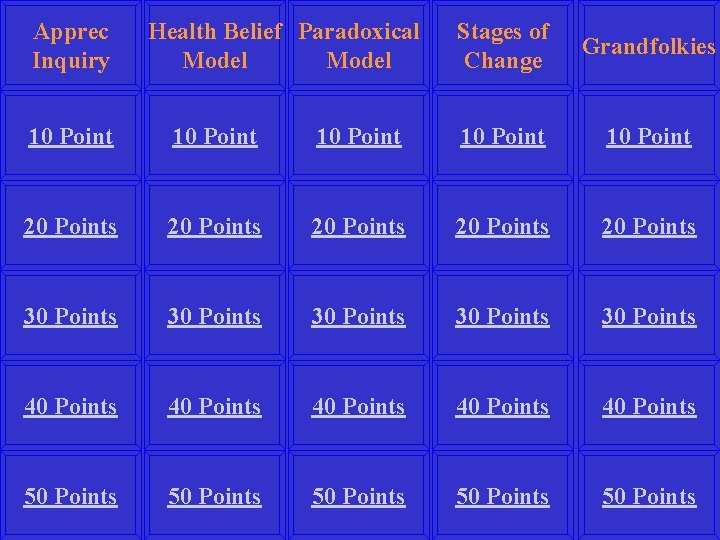
Apprec Inquiry Health Belief Paradoxical Model Stages of Change Grandfolkies 10 Point 10 Point 20 Points 20 Points 30 Points 30 Points 40 Points 40 Points 50 Points 50 Points

Appreciate what is

What is Discovery?

Co-constructing the Ideal
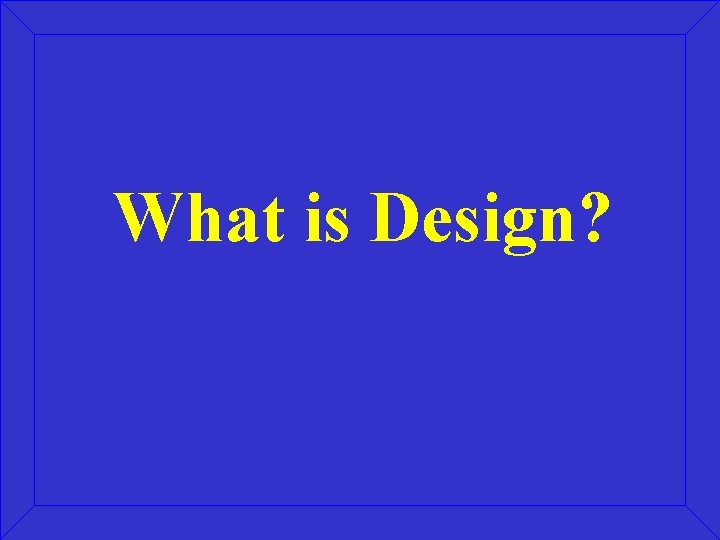
What is Design?

Envisioning Results

What is Dreaming?

Empowers and Sustains

What is Destiny?

Planning Change through positive acknowledgment

What is Appreciative Inquiry?

Believe in Health Strategies

What is Perceived Benefits?

Events that motivate

What are Cues to Action?

Potential Negative Consequences

What are Perceived Barriers?

See self at risk for contracting an illness

What is Perceived Susceptibility?

Perceived Susceptibility & Perceived Severity

What is Perceived Threat?

Father of Gestalt Therapy

Who was Fritz Perls?

Being what one is at the moment

What is Gestalt?

Has roots in Psychotherapy & Phenomenology

What is theory of Paradoxical Change?

Takes a dialogic Wholistic approach

What is a Gestalt change agent (or therapist)?

Provides an orderly model for social change

What is the Paradoxical Change Model?

No intention to change

What is Precontemplation?

Works to maintain the change

What is Adaptation - Maintenance?

Devotes time, energy and develops strategies

What is Action?

Time to set goals and priorities

What is Preparation?

Aware of problem but not commited to change

What is Contemplation?

Set Clear Goals

What is Planning?

Dealing with Resistance

What is Transitions?

Conduct a Reality Check

What is Visioning?

Relax and Reflect on Progress

What is Evaluation?

Converting Plans to Reality

What is Evaluation?

Make your wager
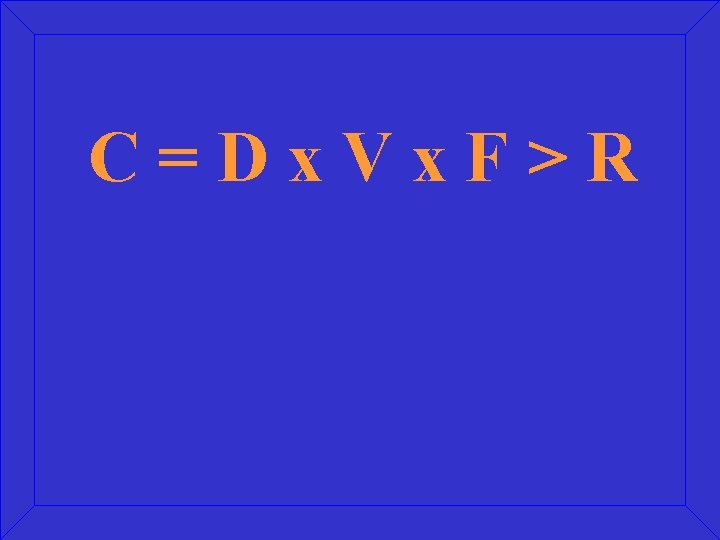
C=Dx. Vx. F>R
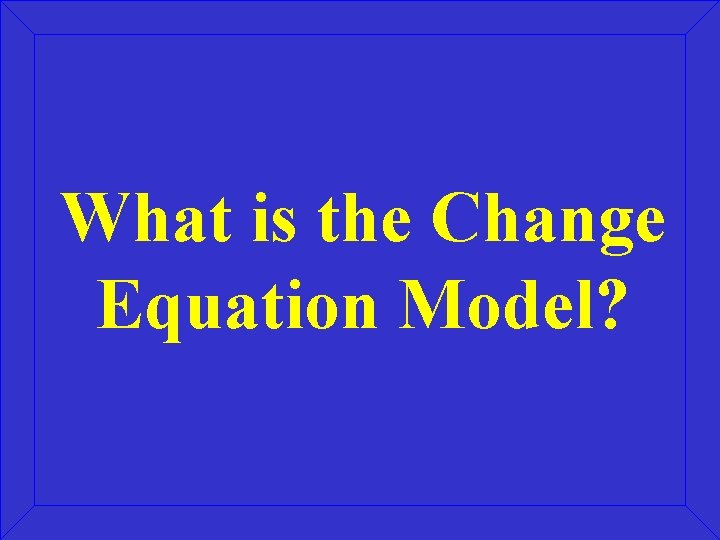
What is the Change Equation Model?
- Slides: 56