Cholinesterase Inhibitors and Their Use in Myasthenia Gravis

Cholinesterase Inhibitors and Their Use in Myasthenia Gravis

Cholinesterase Inhibitors Drugs that prevent the degradation of acetylcholine (ACh) by acetylcholinesterase Viewed as indirect-acting cholinergic agonists Lack selectivity (muscarinic, ganglionic, and neuromuscular) Limited therapeutic applications
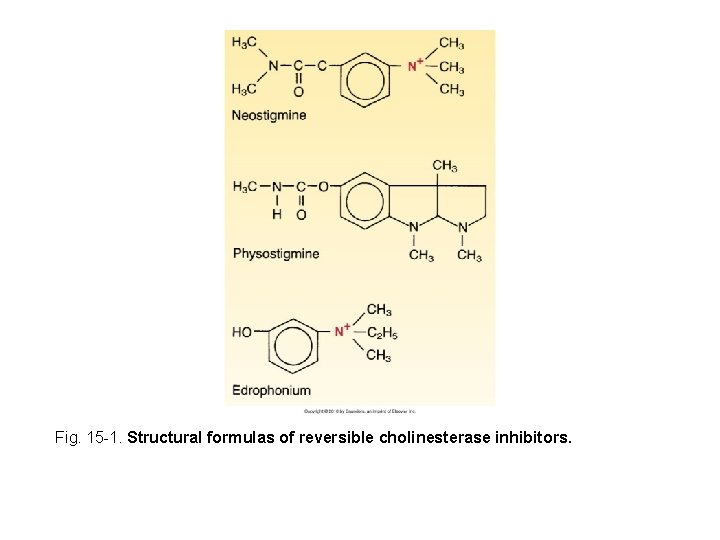
Fig. 15 -1. Structural formulas of reversible cholinesterase inhibitors.
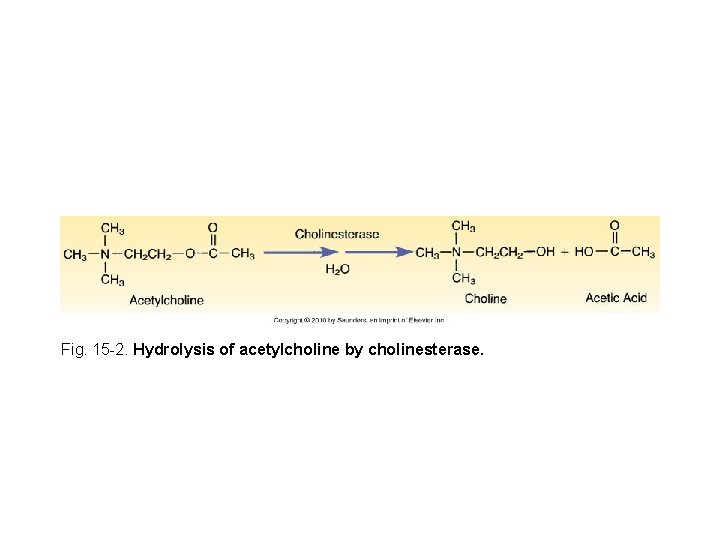
Fig. 15 -2. Hydrolysis of acetylcholine by cholinesterase.
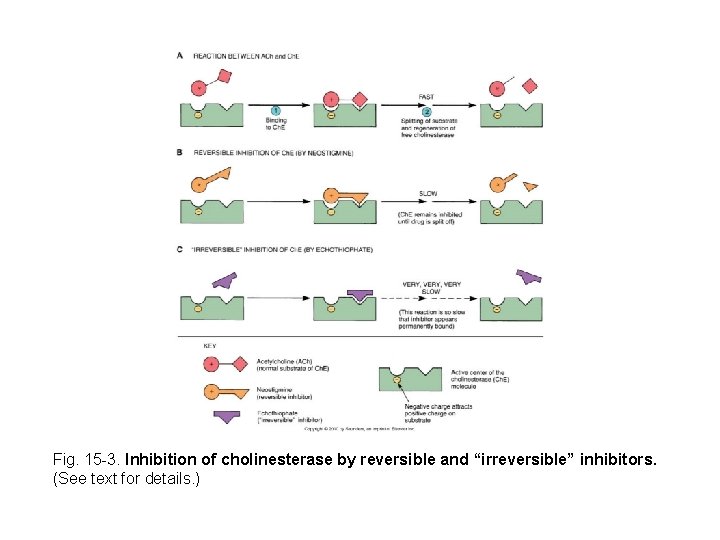
Fig. 15 -3. Inhibition of cholinesterase by reversible and “irreversible” inhibitors. (See text for details. )

Cholinesterase Inhibitors “Reversible” cholinesterase inhibitors Ø Ø Neostigmine Other reversible cholinesterase inhibitors “Irreversible” cholinesterase inhibitors Ø Ø Basic pharmacology Toxicology

“Reversible” Cholinesterase Inhibitors Neostigmine (Prostigmin) Ø Ø Cannot readily cross membranes Absorbed poorly with oral administration Minimal effects on brain and fetus Poor substrate for cholinesterase (Ch. E)

Neostigmine (Prostigmin) Mechanism of action Ø Pharmacologic effects • Therapeutic administration: muscarinic receptors Ø Muscarinic responses • Identical to muscarinic agonist response
Neostigmine (Prostigmin) Mechanism of action Ø Neuromuscular effects • Therapeutic dose: increases force of contraction in skeletal muscle • Toxic levels: decrease force of contraction Ø Central nervous system • Therapeutic levels: mild stimulation • Toxic levels: depress the CNS

Neostigmine (Prostigmin) Therapeutic uses Ø Ø Myasthenia gravis Reversal of nondepolarizing neuromuscular blockade • Used postoperatively • Treatment of overdose • Likely to elicit substantial muscarinic responses • May need to administer atropine (muscarinic antagonist)

Neostigmine (Prostigmin) Adverse effects/acute toxicity Ø Ø Ø Excessive muscarinic stimulation Neuromuscular blockade Treatment with antagonist Precautions and contraindications Ø Ø Ø Obstruction of GI or urinary tract Peptic ulcer disease Asthma Coronary insufficiency Hyperthyroidism
Neostigmine (Prostigmin) Drug interactions Ø Ø Ø Muscarinic antagonists Nondepolarizing neuromuscular blockers Depolarizing neuromuscular blockers

Other “Reversible” Cholinesterase Inhibitors Physostigmine Ambenonium, edrophonium, and pyridostigmine Echothiophate Drugs for Alzheimer’s disease

“Irreversible” Cholinesterase Inhibitors Highly toxic Primarily used as insecticides Only clinical application is glaucoma All contain an atom of phosphorus Almost all are highly lipid soluble Readily absorbed from several routes Potential use in chemical warfare

“Irreversible” Cholinesterase Inhibitors Toxicology Sources of poisoning Symptoms • Cholinergic crisis Ø Treatment • Mechanical ventilation • Pralidoxime • Diazepam Ø Pralidoxime • Specific antidote to poisoning • Effectiveness impacted by early administration Ø Ø
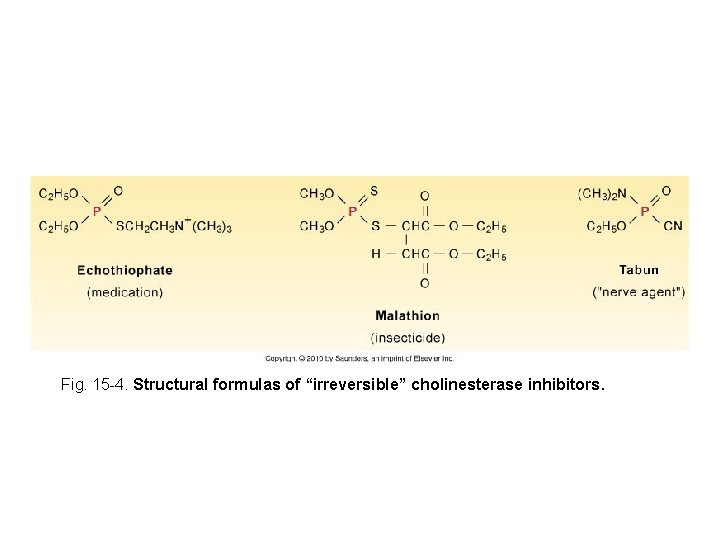
Fig. 15 -4. Structural formulas of “irreversible” cholinesterase inhibitors.

Myasthenia Gravis Pathophysiology Characterized by fluctuating muscle weakness and predisposition to rapid fatigue Ø Common symptoms • Ptosis, dysphagia, weakness of skeletal muscles Ø Autoimmune process in which antibodies attack nicotinic. M receptors on skeletal muscle Ø

Myasthenia Gravis Treatment with cholinesterase inhibitors Beneficial effects • Increased muscle strength Ø Side effects • Excessive muscarinic response Ø Dosage adjustment • Start small and adjust to patient response • May need to modify dosage in anticipation of exertion • Signs of undermedication Ø Ptosis, difficulty in swallowing Excessive salivation and other muscarinic responses • Signs of overmedication

Myasthenia Gravis Myasthenic crisis and cholinergic crisis Ø Cholinergic crisis • Characterized by extreme muscle weakness or frank paralysis and signs of excessive muscarinic stimulation • Treatment with respiratory support and atropine Ø Distinguishing myasthenic crisis from cholinergic crisis • History of medication use or signs of excessive muscarinic stimulation assist with differential diagnosis. Use of identification by the patient
- Slides: 19