Chiroptical Spectroscopy Compiled by Krisztina Pl 1 Introduction
Chiroptical Spectroscopy Compiled by Krisztina Pál 1
Introduction • Chiroptical spectroscopic methods enable us to distinguish enantiomeric/diastereomeric forms of chiral compounds – Polarimetry (optical rotation at a selected wavelength), – ORD /optical rotation dispersion/ spectroscopy (optical rotation as a function of wavelength) – CD /circular dichroism spectroscopy/ (difference of the absorption of light polarized circularly left-handed and righthanded) • Chiroptical spectroscopic methods are based upon the interaction between the optically active sample and the polarized light. 2

Optically active or chiral materials • capable of rotating the plane of vibration of polarized light to the right - Arago, quartz crystal (1811) – Biot, tartaric acid aqueous solution (1838) – Le Bel és van't Hoff (1874); asymmetric, tetrahedral C-atom 3

Aimé Cotton (1869 - 1951) discovered Optical rotatory dispersion = ORD Circular dichroism = CD spectroscopic methods for the determination of stereo structures of chiral compounds 4
Chiral: not superimposable on its mirror image. Origin of word ‚chiral’ is kheir meaning hand in Greek. Such mirror structures are related as right and left hands. Optical activity = the plane of polarization of linearly polarized light is rotated as it travels through certain materials; samples of chiral molecules are optically active (except racemic mixtures) 5
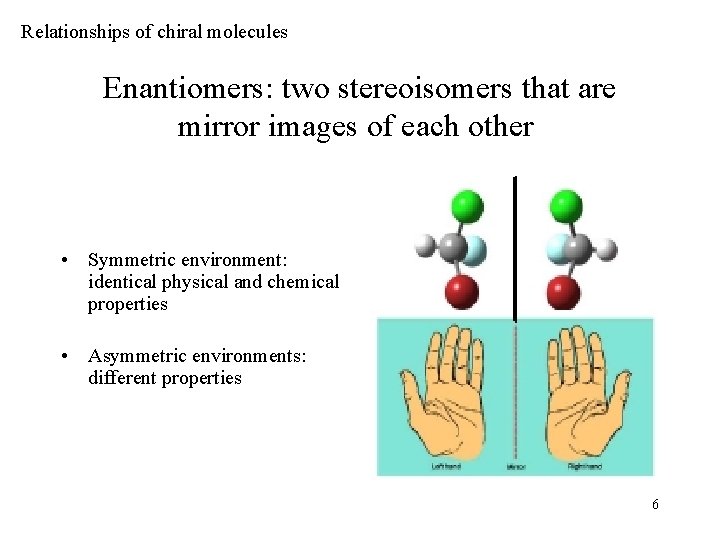
Relationships of chiral molecules Enantiomers: two stereoisomers that are mirror images of each other • Symmetric environment: identical physical and chemical properties • Asymmetric environments: different properties 6
Properties of enantiomers • Melting point, boiling point, indece of refraction, solubilities – same values • Identical UV-VIS, IR, NMR spectra • Different behavior in interaction with chiral agents: – Different solubilities in chiral solvents – Different reactivities with chiral reagents (diastereomer salts, basis of resolvation) – Different interaction with ‚chiral’ light • (diastereomers: isomers of an optically active compounds with two or more stereocenters. The configuration of at least one center is identical, at least one is different. ) 7
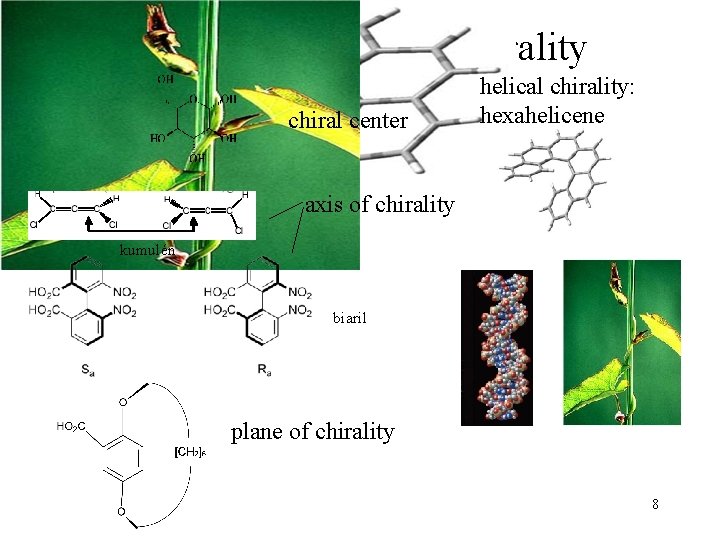
Types of molecular chirality chiral center helical chirality: hexahelicene axis of chirality kumulén biaril plane of chirality 8
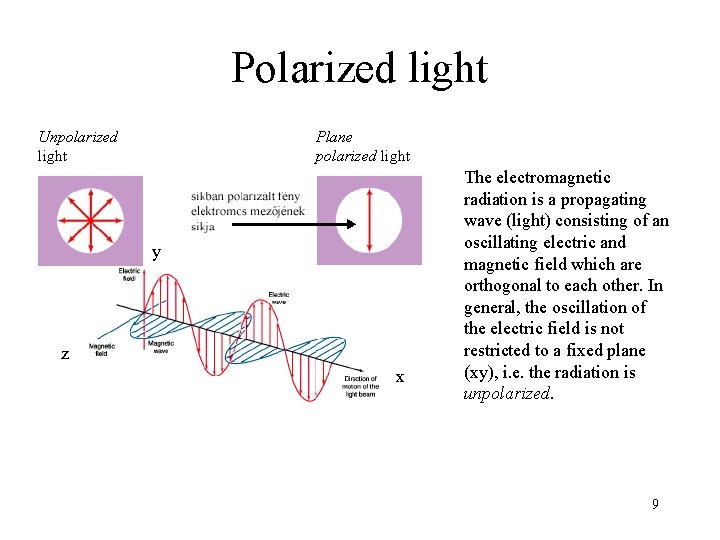
Polarized light Unpolarized light Plane polarized light y z x The electromagnetic radiation is a propagating wave (light) consisting of an oscillating electric and magnetic field which are orthogonal to each other. In general, the oscillation of the electric field is not restricted to a fixed plane (xy), i. e. the radiation is unpolarized. 9
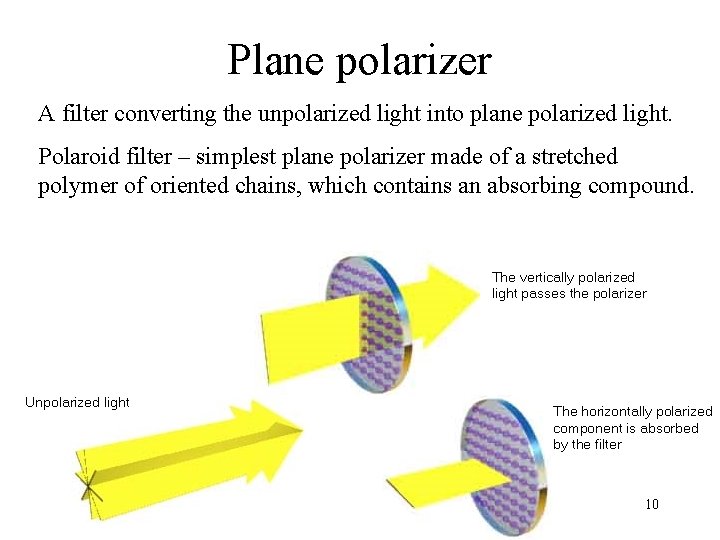
Plane polarizer A filter converting the unpolarized light into plane polarized light. Polaroid filter – simplest plane polarizer made of a stretched polymer of oriented chains, which contains an absorbing compound. The vertically polarized light passes the polarizer Unpolarized light The horizontally polarized component is absorbed by the filter 10

Optical rotation • The index of refraction of a chiral sample is different for the two components of plane polarized light (right- and left -handed circularly polarized light) • nleft≠nright • Thus, the velocities of the two circularly polarized components are different, as the plane polarized light passes through the chiral sample • n=c/v • Consequence: the plane of polarization of the plane polarized light rotates (by angel ) 11

Polarimetry: Measuring the angle of rotation of the plane of polarization at a defined wavelength /589 nm (Na D line)/ 12
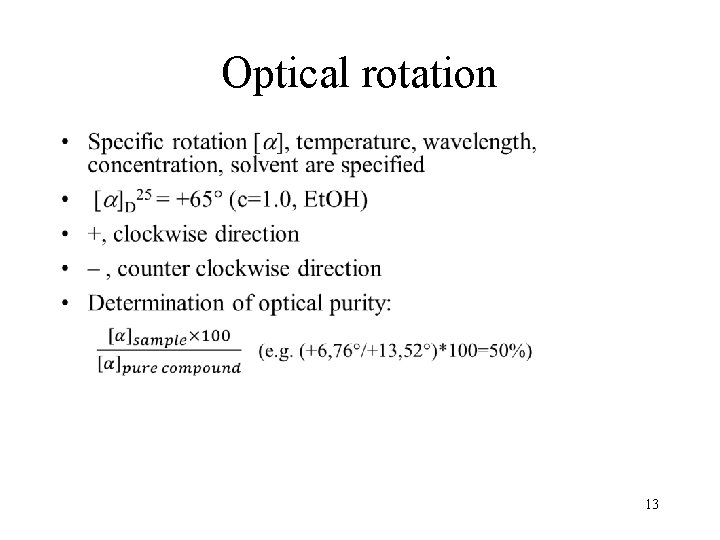
Optical rotation • 13

Optical rotatory dispersion (ORD) • The optical rotation as a function of wavelength (dispersion = dependence on wavelength) Detector (photoelectron multiplier) Light source (lamp + diffraction grating) 14
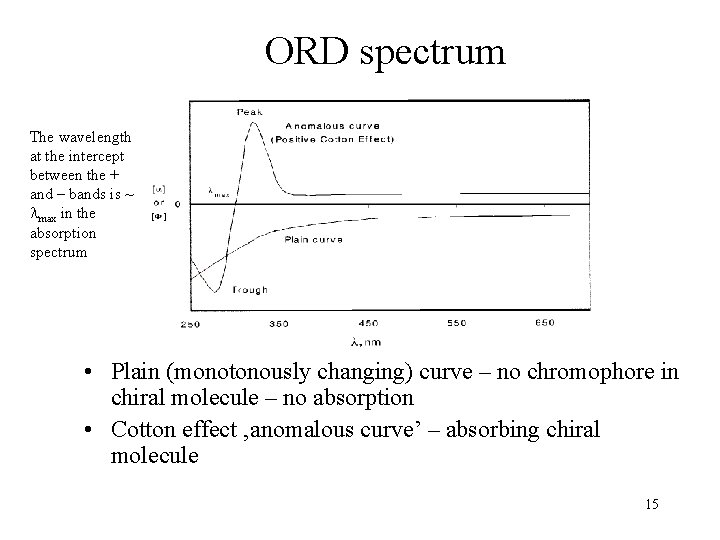
ORD spectrum The wavelength at the intercept between the + and – bands is ~ max in the absorption spectrum • Plain (monotonously changing) curve – no chromophore in chiral molecule – no absorption • Cotton effect ‚anomalous curve’ – absorbing chiral molecule 15
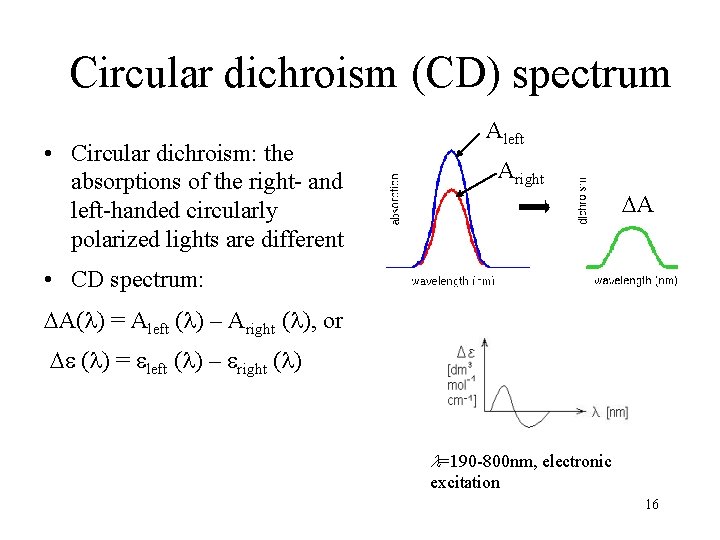
Circular dichroism (CD) spectrum • Circular dichroism: the absorptions of the right- and left-handed circularly polarized lights are different Aleft Aright A • CD spectrum: A( ) = Aleft ( ) – Aright ( ), or ( ) = left ( ) – right ( ) l=190 -800 nm, electronic excitation 16
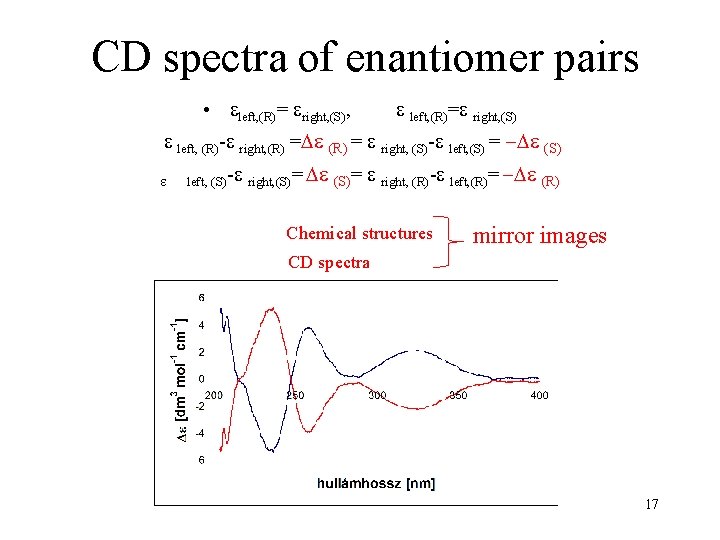
CD spectra of enantiomer pairs • left, (R)= right, (S), left, (R)= right, (S) left, (R)- right, (R) = right, (S)- left, (S) = (S) left, (S)- right, (S)= right, (R)- left, (R)= (R) Chemical structures mirror images CD spectra 17
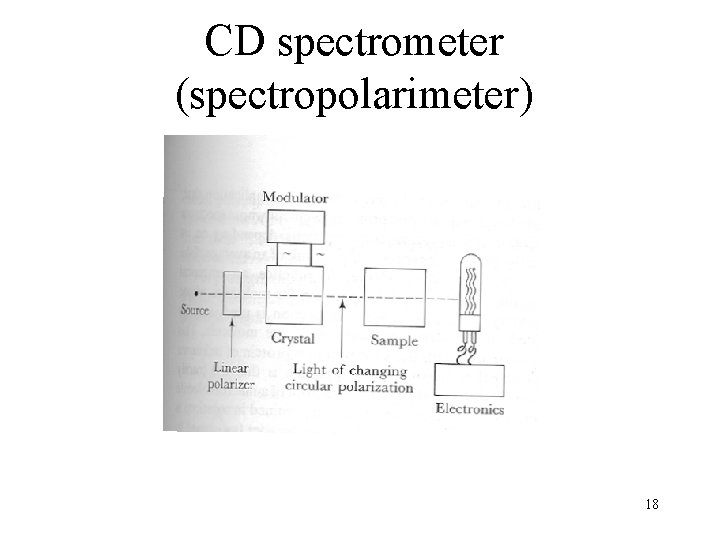
CD spectrometer (spectropolarimeter) 18
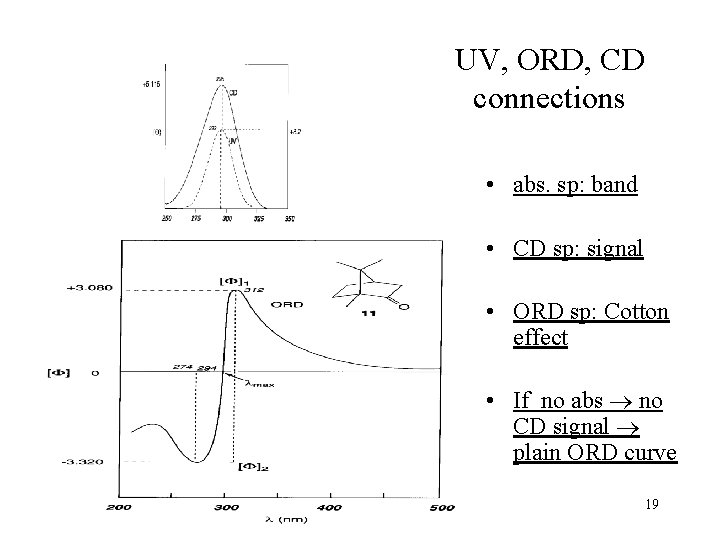
UV, ORD, CD connections • abs. sp: band • CD sp: signal • ORD sp: Cotton effect • If no abs no CD signal plain ORD curve 19
Applications of CD spectroscopy • Determination of enantiomer purity • Determination of absolute configuration • Induced CD signal: binding of an achiral molecule to a chiral molecule – CD bands in the absorption range of the achiral molecule may appear • Protein CD spectra: secondary structure. conformational changes 20
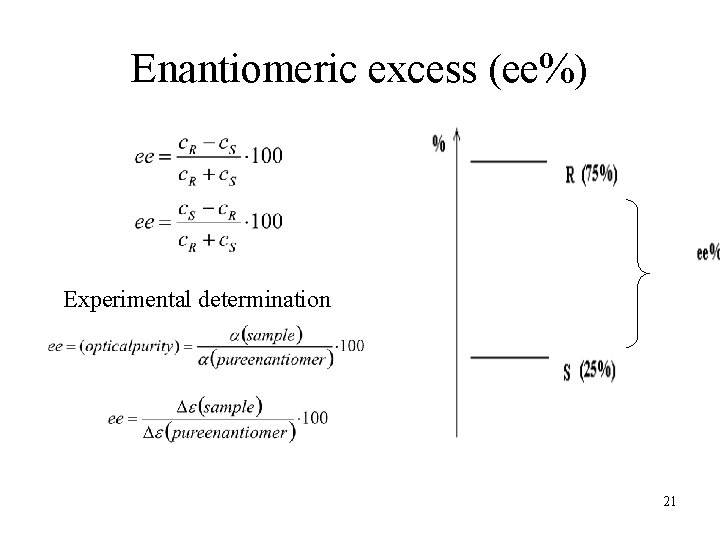
Enantiomeric excess (ee%) Experimental determination 21
Importance of optical purity of chiral drugs • 40% of currently produced drugs are chiral compounds, many of wich are distributed in form of racemic mixtures • The pharmacokinetics of enantiomeric pairs can be different, usually they act on different receptors • In many cases only one of the enantiomers provides the desired clinical effect, the other enantiomer is uneffective or has adverse effects 22

Contergan-scandal (thalidomide) Thalidomide is a chiral drug molecule, marketed first in 1957, under brand name Contergan • It was an efficient sedative to cure panic disorder, anxiety, sleeplesness, psychic traumas and also nausea of pregnant women • Many babies were born with limb defiencies 23
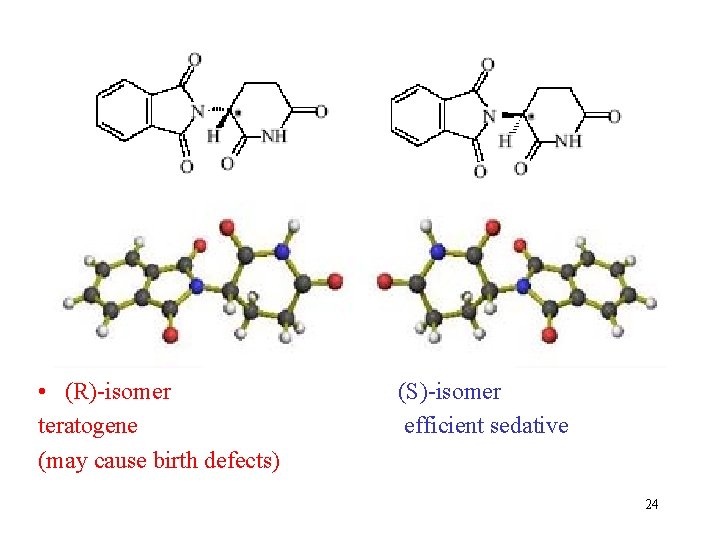
• (R)-isomer teratogene (may cause birth defects) (S)-isomer efficient sedative 24
Determination the absolute configuration by CD spectroscopy • (1): Comparison of CD spectra: Compound with unknown configuration similar compound with known configuration: CD spectra are similar are close to mirror images • (2): Empirical rules: The sign of some CD signals can be realted to the absolute configuration (e. g. octant rule) • (3): Quantum chemical calculation of CD spectrum and comparison of the experimental and theoretically calculated spectra 25
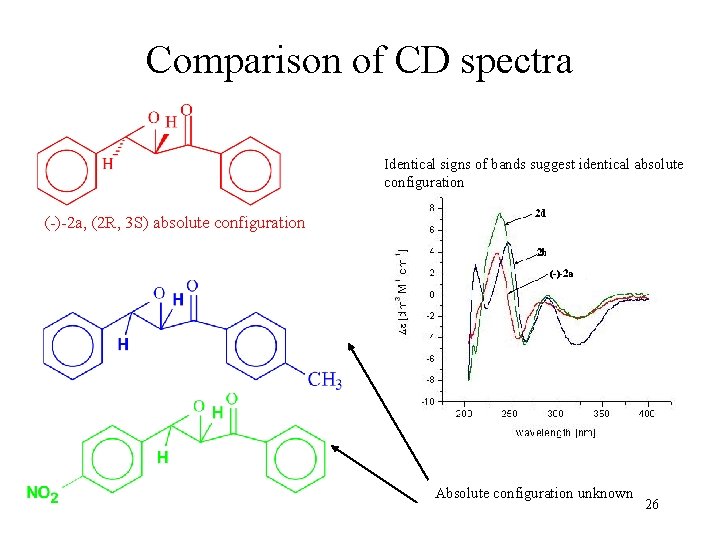
Comparison of CD spectra Identical signs of bands suggest identical absolute configuration (-)-2 a, (2 R, 3 S) absolute configuration Absolute configuration unknown 26

Octant rule for ketones • n→π* transitions of C=O groups falls to ~ 300 nm. It produces a weak band in UV abs. spectrum and a stronger band in CD spectrum. The sign of the CD band is related to the abs. conf. of chiral ketones The sign of the octant including the majority of the atoms and the sign of the C=O band in the CD spectrum will be the same. The 3 D model of the molecule is arranged in a coordinate system, with the center of the C=O bond in the origin 27
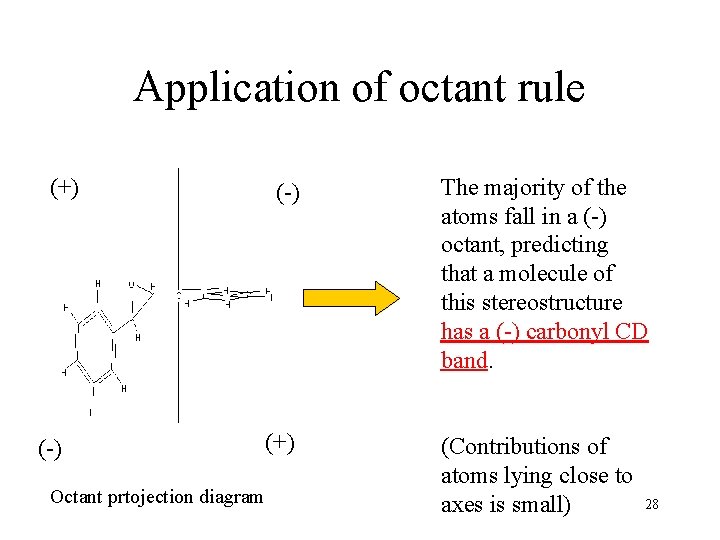
Application of octant rule (+) (-) Octant prtojection diagram (-) The majority of the atoms fall in a (-) octant, predicting that a molecule of this stereostructure has a (-) carbonyl CD band. (+) (Contributions of atoms lying close to 28 axes is small)
Induced CD effect • Chiral host (e. g. cyclodextrin) or binding site (enzyme, DNS) • Achiral, chromophore guest (dye probe, drug molecule) • Achiral chromophore may become distrorted in a chiral structure or its electronic transitions are chirally perturbed – a CD signal is induced in the absorption range of the chromophore 29
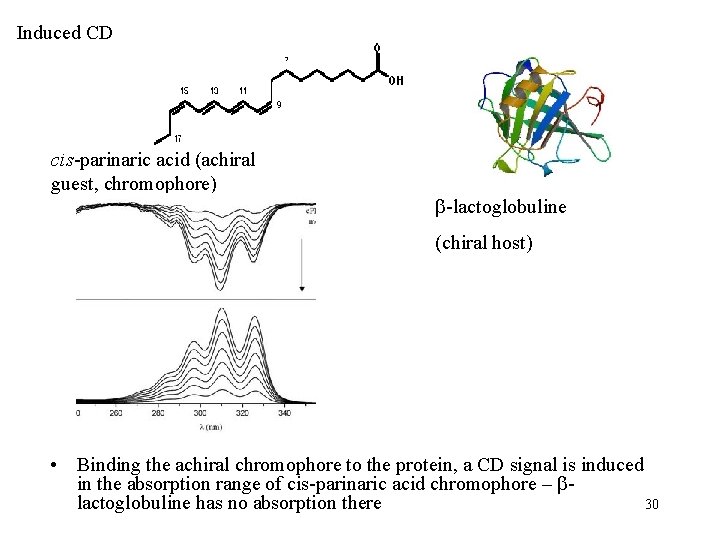
Induced CD cis-parinaric acid (achiral guest, chromophore) -lactoglobuline (chiral host) • Binding the achiral chromophore to the protein, a CD signal is induced in the absorption range of cis-parinaric acid chromophore – lactoglobuline has no absorption there 30
CD spectroscopy of proteins • Environmental changes (p. H, temperature, etc. ) → conformation changes • CD spectrum sensitive to conformation • CD spektroscopy is a key technique in protein studies (denaturation studies, protein binding studies) 31
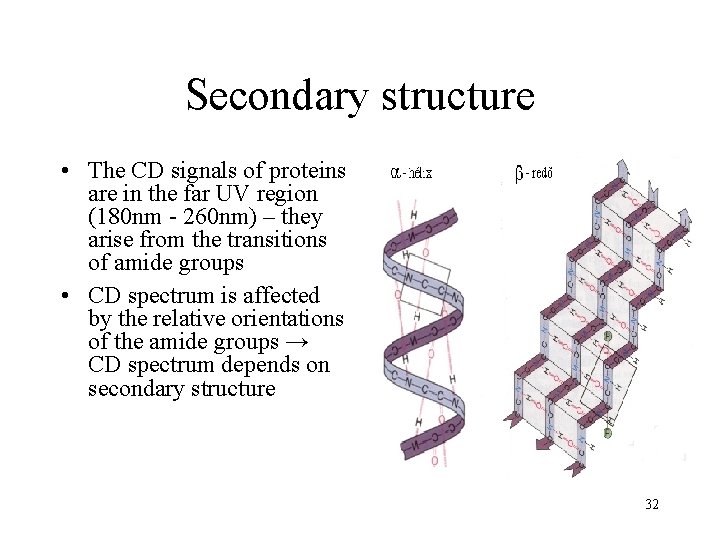
Secondary structure • The CD signals of proteins are in the far UV region (180 nm - 260 nm) – they arise from the transitions of amide groups • CD spectrum is affected by the relative orientations of the amide groups → CD spectrum depends on secondary structure 32

CD spectra of ‚pure’ secondary structures -helix disordered structure -turn -sheet • The CD spectrum of a protein can be considered the linear combination of the spectra of secondary structures 33
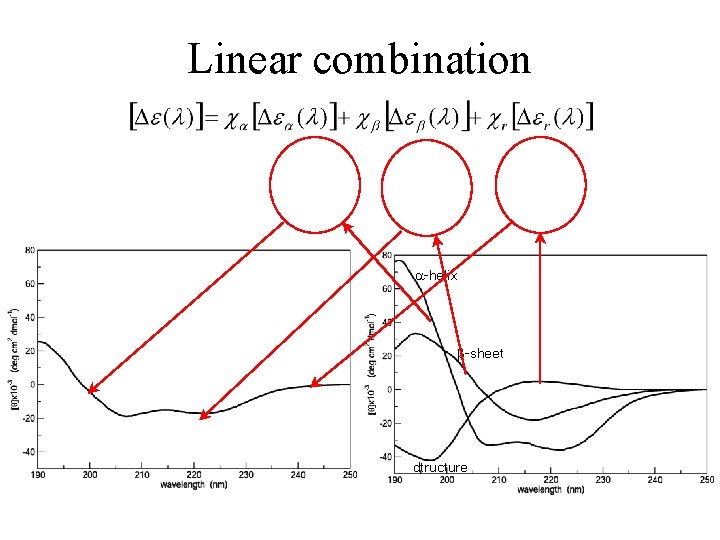
Linear combination -helix -sheet dtructure 34

Thank you for the attention! 35
- Slides: 35