CHIRAL RECOGNITION IN NEUTRAL AND IONIC MOLECULAR COMPLEXES







- Slides: 19

CHIRAL RECOGNITION IN NEUTRAL AND IONIC MOLECULAR COMPLEXES Ananya Sen, Aude Bouchet, Valeria Lepere, Katia Le Barbu Debus, Anne Zehnacker Rentien Institut des Sciences Moléculaires d’Orsay ISMO CNRS-Université Paris Sud Orsay-France International Symposium on Molecular Spectroscopy - 68 th Meeting (June 17 -21 2013) 1

INTRODUCTION An object that cannot be superimposed on its mirror image is called chiral. S R R(+)-limonene Turpentine odour S(-)-limonene Orange aroma Mirror plane 2

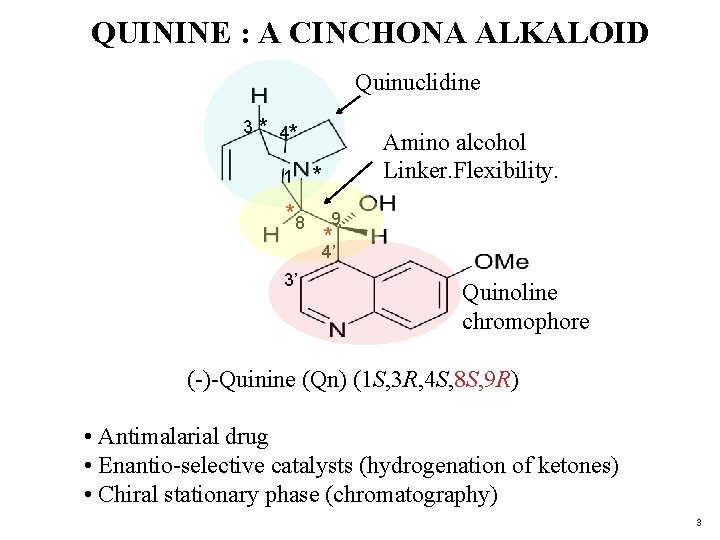
QUININE : A CINCHONA ALKALOID Quinuclidine 3 * 4* 1 *8 Amino alcohol Linker. Flexibility. * 9 * 4’ 3’ Quinoline chromophore (-)-Quinine (Qn) (1 S, 3 R, 4 S, 8 S, 9 R) • Antimalarial drug • Enantio-selective catalysts (hydrogenation of ketones) • Chiral stationary phase (chromatography) 3

QUININE: A PSEUDOENANTIOMER * * * 8* 9 PSEUDOENANTIOMERS (-)Quinine (Qn) (1 S, 3 R, 4 S, 8 S, 9 R) * * 9 * 8 (+)Quinidine (Qd)(1 S, 3 R, 4 S, 8 R, 9 S) • slightly different packing properties in the crystal. • slightly different efficiency against malaria interpreted in terms of different solubility. 4

PHOTOPHYSICS OF QUININE hn Protonated Quinine used as a fluorescence standard. (Qf =0. 546 for exc=310 nm) hn H O H n p* Neutral quinine Nonpolar solvents Charge Transfer low Qf ¹Albert M Brouwer J. Phys. Chem. C 2009, 113, 11790 -11795 p p* Protonated/H-bonded quinine ¹ Addition of water/ protonation no charge transfer. Locally Excited (high Qf) emission restored 5

OBJECTIVE ü The spectroscopic properties of jet-cooled Quinine in gas phase. ü Comparison with Vibrational Circular Dichroism studies of Quinine in solution. 6

TECHNIQUES In the gas phase: §Laser ablation and Supersonic Expansion § Resonance Enhanced Multiphoton Ionisation (REMPI) § Laser Induced Fluorescence Spectroscopy (LIF) § IR-UV Double Resonance Spectroscopy In solution: § Vibrational Circular Dichroism 7

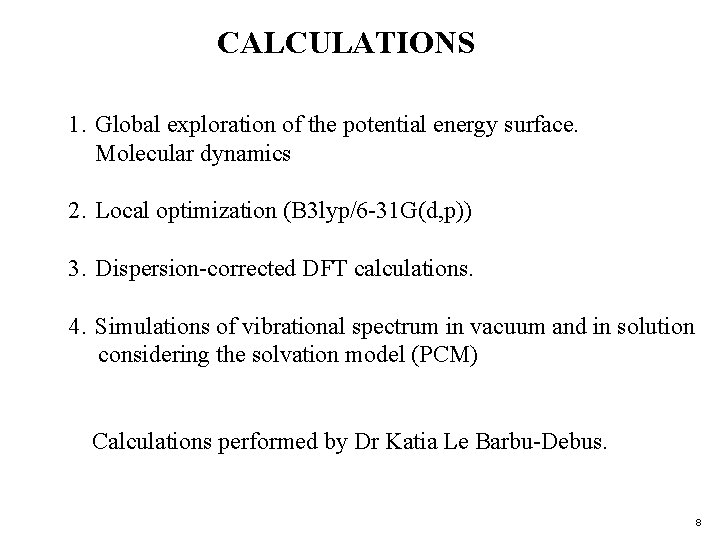
CALCULATIONS 1. Global exploration of the potential energy surface. Molecular dynamics 2. Local optimization (B 3 lyp/6 -31 G(d, p)) 3. Dispersion-corrected DFT calculations. 4. Simulations of vibrational spectrum in vacuum and in solution considering the solvation model (PCM) Calculations performed by Dr Katia Le Barbu-Debus. 8

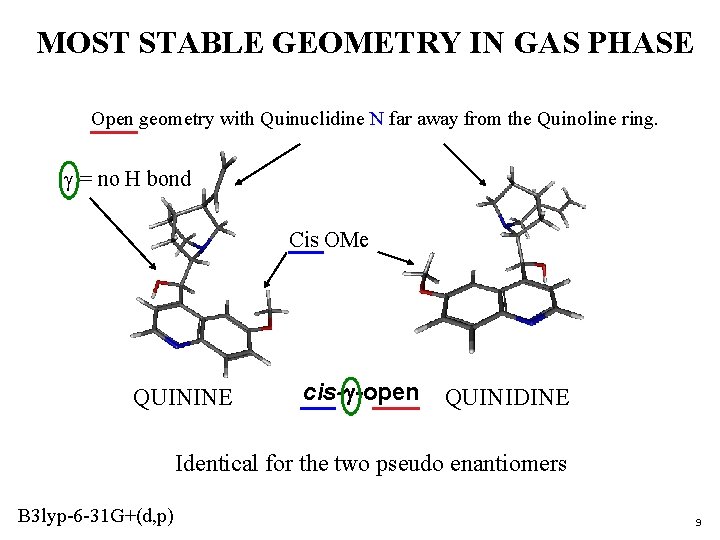
MOST STABLE GEOMETRY IN GAS PHASE Open geometry with Quinuclidine N far away from the Quinoline ring. g = no H bond Cis OMe QUININE cis-g-open QUINIDINE Identical for the two pseudo enantiomers B 3 lyp-6 -31 G+(d, p) 9

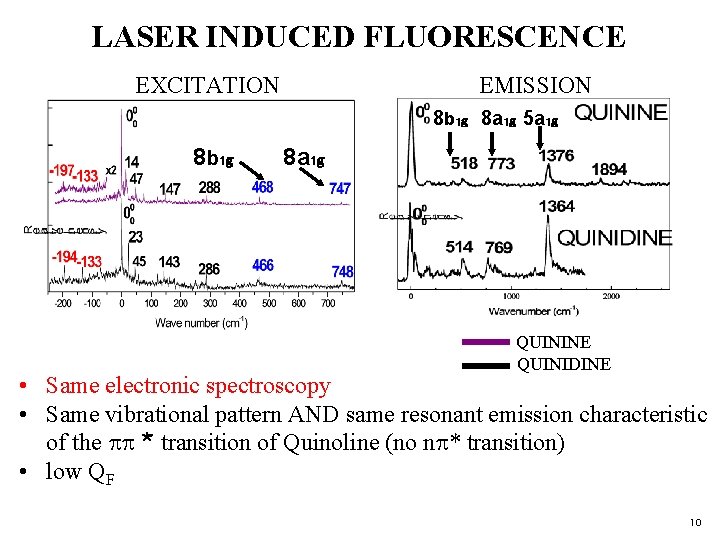
LASER INDUCED FLUORESCENCE EXCITATION EMISSION 8 b₁g 8 a₁g 5 a₁g 8 b ₁g 8 a₁g QUININE QUINIDINE • Same electronic spectroscopy • Same vibrational pattern AND same resonant emission characteristic of the pp * transition of Quinoline (no np* transition) • low QF 10

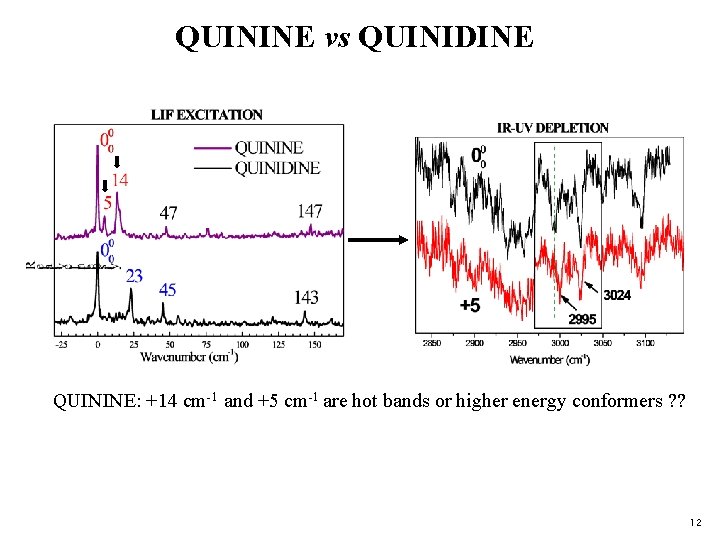
QUININE vs QUINIDINE Different (CH) Probe kept on 0 -0 bend Chiral linker Same (OH) bend OMe QUINIDINE: Probe kept on 0 -0, +23 cm-1 and +45 cm-1 show no difference. 11

QUININE vs QUINIDINE QUININE: +14 cm-1 and +5 cm-1 are hot bands or higher energy conformers ? ? 12

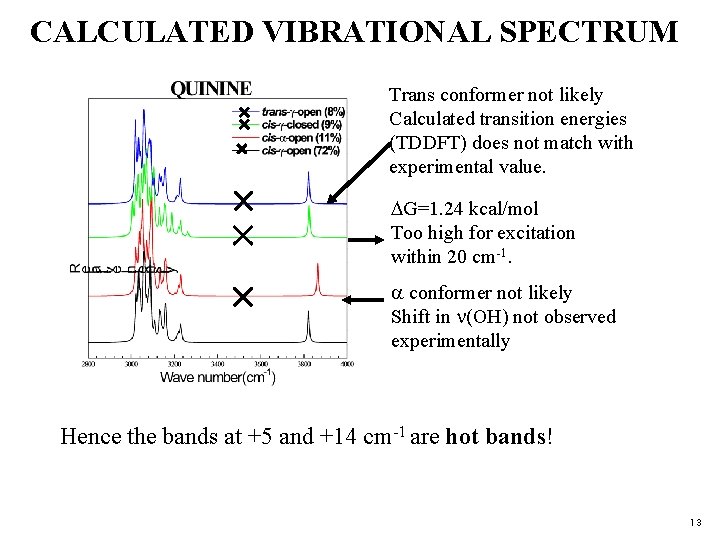
CALCULATED VIBRATIONAL SPECTRUM Trans conformer not likely Calculated transition energies (TDDFT) does not match with experimental value. G=1. 24 kcal/mol Too high for excitation within 20 cm-1. conformer not likely Shift in (OH) not observed experimentally Hence the bands at +5 and +14 cm-1 are hot bands! 13

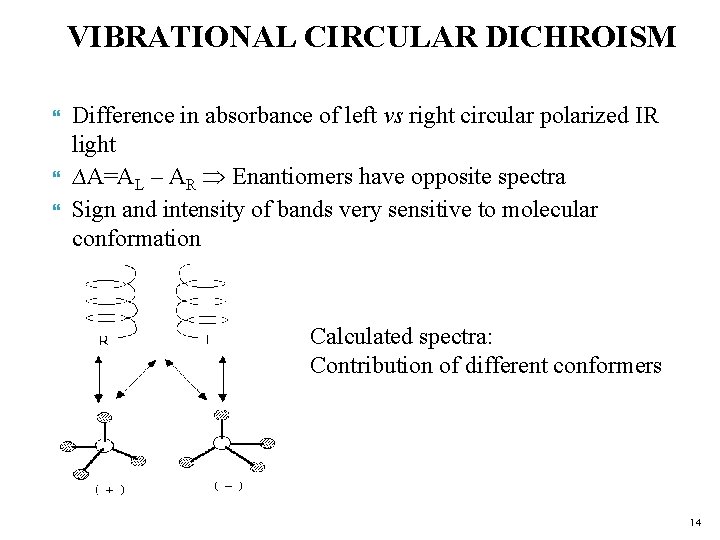
VIBRATIONAL CIRCULAR DICHROISM Difference in absorbance of left vs right circular polarized IR light ∆A=AL – AR Enantiomers have opposite spectra Sign and intensity of bands very sensitive to molecular conformation Calculated spectra: Contribution of different conformers 14

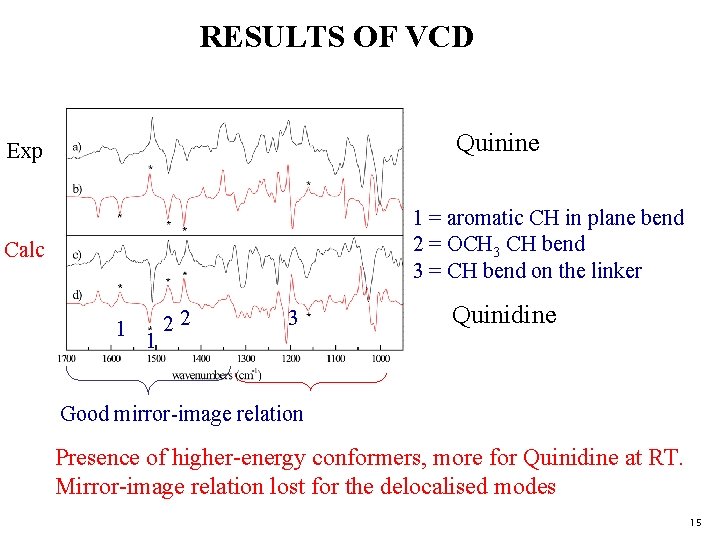
RESULTS OF VCD Quinine Exp 1 = aromatic CH in plane bend 2 = OCH 3 CH bend 3 = CH bend on the linker Calc 1 1 22 3 Quinidine Good mirror-image relation Presence of higher-energy conformers, more for Quinidine at RT. Mirror-image relation lost for the delocalised modes 15

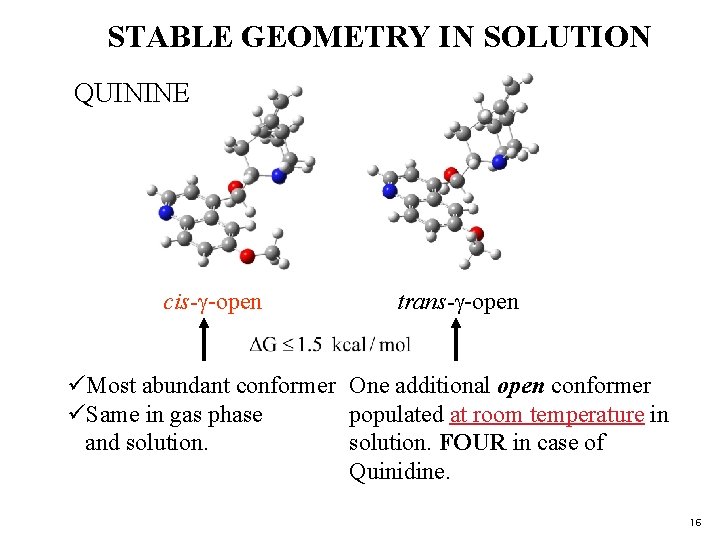
STABLE GEOMETRY IN SOLUTION QUININE cis-g-open trans-g-open üMost abundant conformer One additional open conformer üSame in gas phase populated at room temperature in and solution. FOUR in case of Quinidine. 16

GENERAL CONCLUSION Ø The two pseudo-enantiomers of Quinine do fluoresce in gas phase. Ø Pseudo-enantiomers have very similar properties in the gas phase (same structure). Ø Difference between the two are more prominent in solution than in gas phase. Quinidine is more flexible than Quinine in solution. ² Role of higher energy conformers in Chiral Recognition. ² Sen et. al J. Phys. Chem. A 2012, 116(32), 8334 -8344 17

ACKNOWLEDGEMENTS Dr Anne Zehnacker-Rentien Dr Philippe Bréchignac Dr Katia Le Barbu-Debus Dr Valeria Lepère Dr Debora Scuderi Dr Aude Bouchet And the helpful mechanics and electronic workshop team at ISMO. THANK YOU FOR YOUR ATTENTION 18

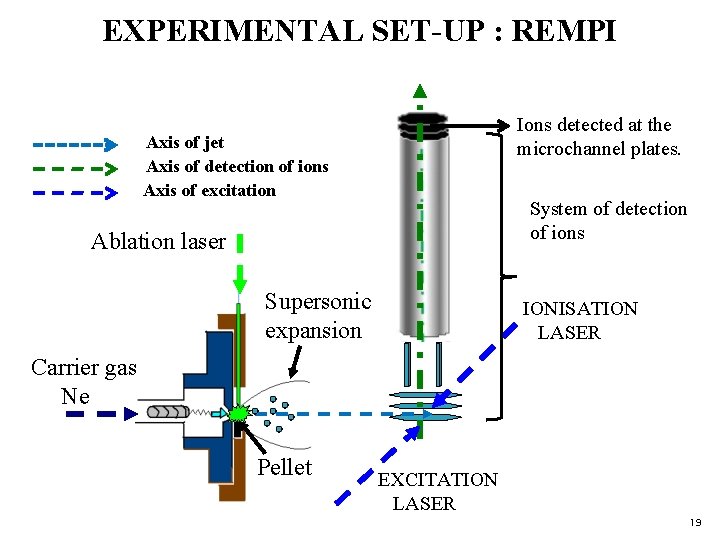
EXPERIMENTAL SET-UP : REMPI Ions detected at the microchannel plates. Axis of jet Axis of detection of ions Axis of excitation System of detection of ions Ablation laser Supersonic expansion IONISATION LASER Carrier gas Ne Pellet EXCITATION LASER 19